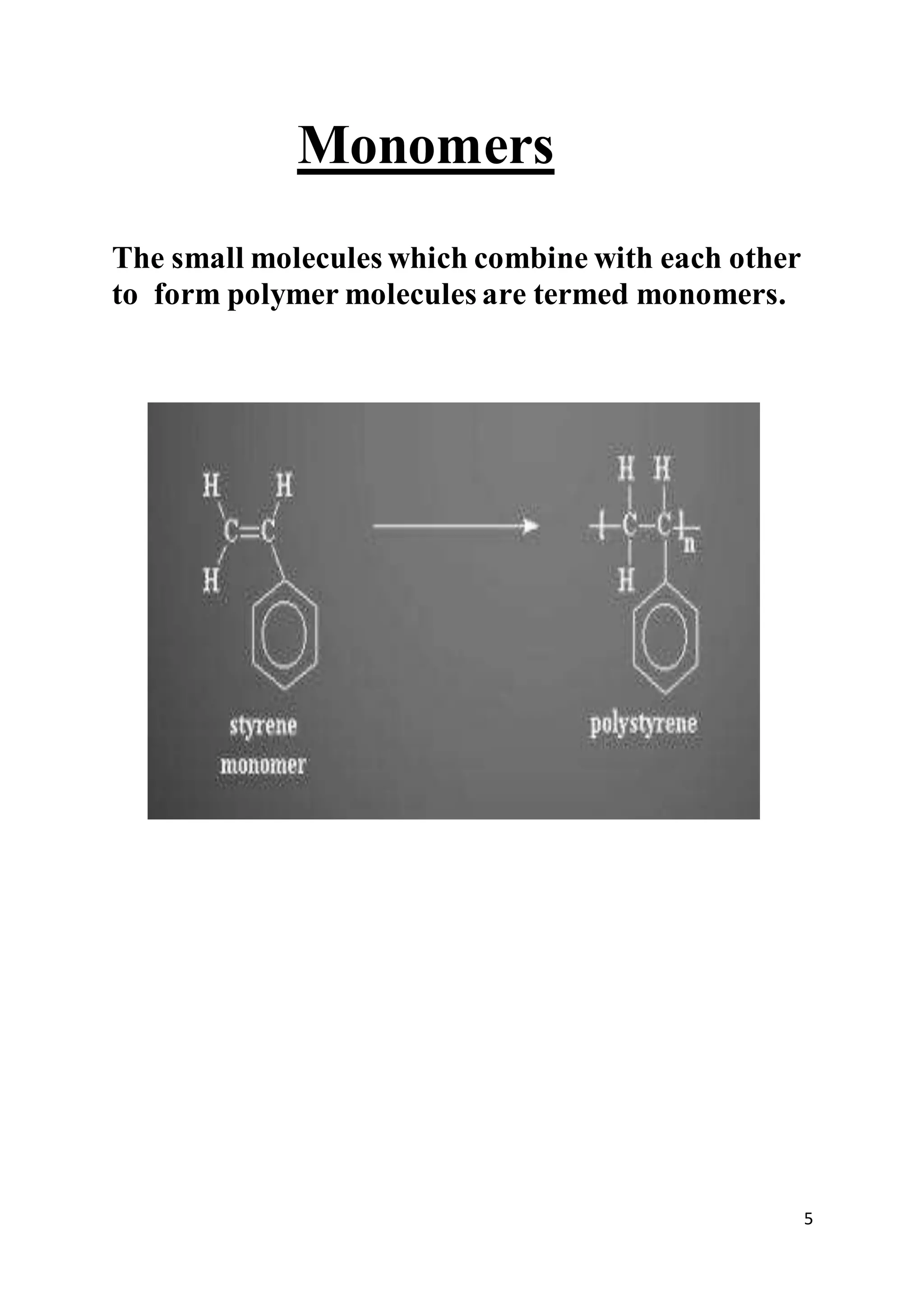
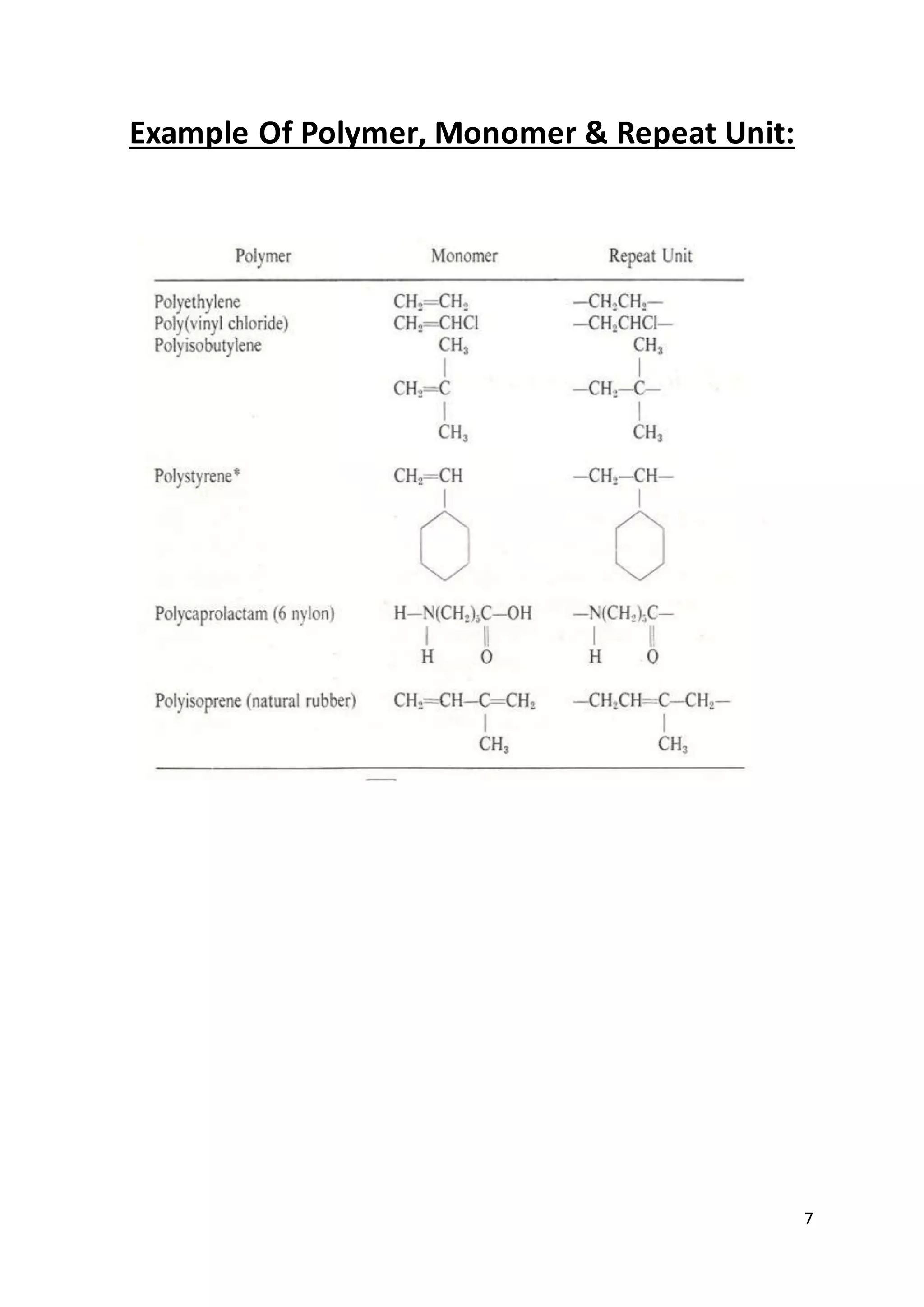
1. The document discusses polymers, polymerization techniques, and addition polymerization. It defines key terms like monomers, repeat units, and classification of polymers by structure.
2. Addition polymers are formed without eliminating small molecules, in contrast to condensation polymers. Addition polymerization occurs via a free radical chain reaction mechanism involving initiation, propagation, and termination steps.
3. Polyethylene is provided as an example of an addition polymer formed from the monomer ethylene using a free radical initiator like benzoyl peroxide in a chain reaction to link monomer units.




![6
Repeat Unit
A repeatunit or repeatingunit is a part of a polymer whose
repetition would producethe complete polymerchain (exceptfor
the end-groupsby linking the repeatunits together successively
along the chain,like the beads of a necklace.
A repeatunitis sometimes called a mer or unit."Mer" originates
from the Greek word "meros," which meansa part. The word
polymer derivesits meaning from this, which means "many mers."
A repeatunit(or mer), is not to be confusedwith the
term monomer,which refersto the small molecule from which a
polymer is synthesized.
One of the simplestrepeatunits is that of the addition polymer
polyvinylchloride, -[CH2-CHCl]n-, whose repeatunit is -[CH2-CHCl]-.
In this case the repeatunit has the same atoms as the
monomervinylchloride CH2=CHCl.When the polymer is formed,
the C=C double bond in the monomer is replaced by a C-C single
bond in the polymer repeatunit, which links by two new bonds to
adjoining repeatunits.
In condensationpolymers (seeexamplesbelow),the repeatunit
contains fewer atoms than the monomeror monomers from which
it is formed.
The subscript"n" denotes the degreeof polymerisation,that is, the
number ofunits linked together.The molecularmass of the repeat
unit, MR, is simply the sum of the atomic massesof
the atoms within the repeatunit. The molecularmass of the chain is
just the productnMR.Other than monodisperse polymers,there is
normally a molar mass distribution caused by chains of different
length.
In copolymers there are two or more types of repeatunit, which
may be arranged in alternation,or at random,or in other more
complex patterns.](https://image.slidesharecdn.com/polymerassignment152-23-4325-200715122949/75/Polymer-Reaction-Technique-6-2048.jpg)





![13
Addition polymerization
Self addition of several bifunctional monomers to each
ohter takes place by chain reaction without the elimination of
any simple molecules.
GENERAL REACTION:
n [CH2=CH] - [-CH2-CH-]n
_
Addition polymerization is initiated by small qty of
substance called Initiators.
–
E.g.-Zeigler-Natta catalyst, potassium persulphate ,
dibenzoyl sulphat e.
Condensationpolymerization
Self addition of several bifunctional monomer to each other
takes place accompanying elimination of simple molecules
like H2O,NH3 & HCL
E.g..
Terylene is obtained by condensing terpthalic acid
[HOOC-C6H4-COOH] with ethylene glycol [HO-
C2H4-OH]
Nylon is made by the condensation of adipic acid
[HOOC-(CH2)4-COOH] with hexamethylene diamine
[NH2-(CH2)6-NH2]](https://image.slidesharecdn.com/polymerassignment152-23-4325-200715122949/75/Polymer-Reaction-Technique-13-2048.jpg)
![14
MECHANISM OF ADDITION
POLYMERIZATION:
FREE RADICLE REACTION MECHANISM
Free Radical Mechanism of chain reaction involves 3 stages namely
1. Initiation
2. Propagation
3. Termination
SCHEMATIC REPRESENTATION
[ R* - Free radical
M* - Unsaturated Monomer]
Generation of free radical :
I > 2R E.g. <> CH2=CH2
Initiation :
R+ M <> RM1
Propagation :
RM1+ M <> RM2
RM2+ M <> RM3
RM( x – 1 )+ M <> RM x
RM( y – 1 )+ M <> RM y
Termination :
RM x + RM y <> RM x + y (COUPLING)
RM x + RM y <> RM x + RM y](https://image.slidesharecdn.com/polymerassignment152-23-4325-200715122949/75/Polymer-Reaction-Technique-14-2048.jpg)


