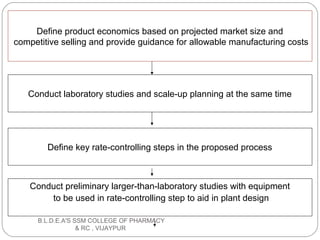
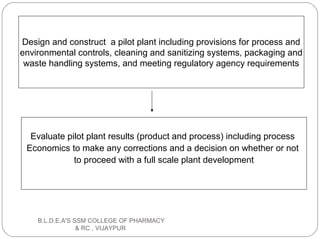
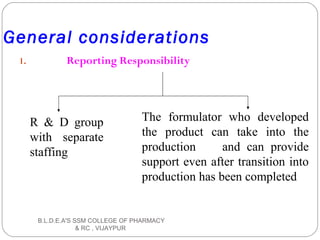
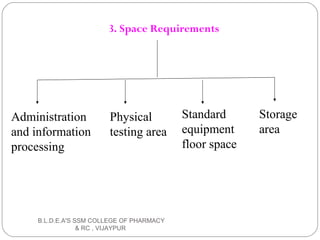
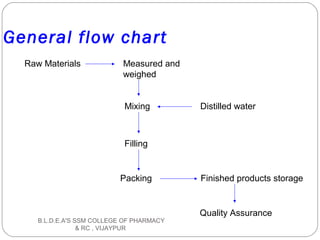
The document discusses pilot plant scale-up techniques. It defines a pilot plant as transforming a lab scale formula into a viable manufacturing process. The objectives of a pilot plant include testing the process before committing funds to full production and examining the formula's ability to withstand scaling. Key steps in scale-up involve defining product economics, conducting lab and planning studies, evaluating rate-controlling steps, designing and constructing a pilot plant, and evaluating results. General considerations for scale-up include personnel requirements, equipment selection, production rates, and process evaluation.