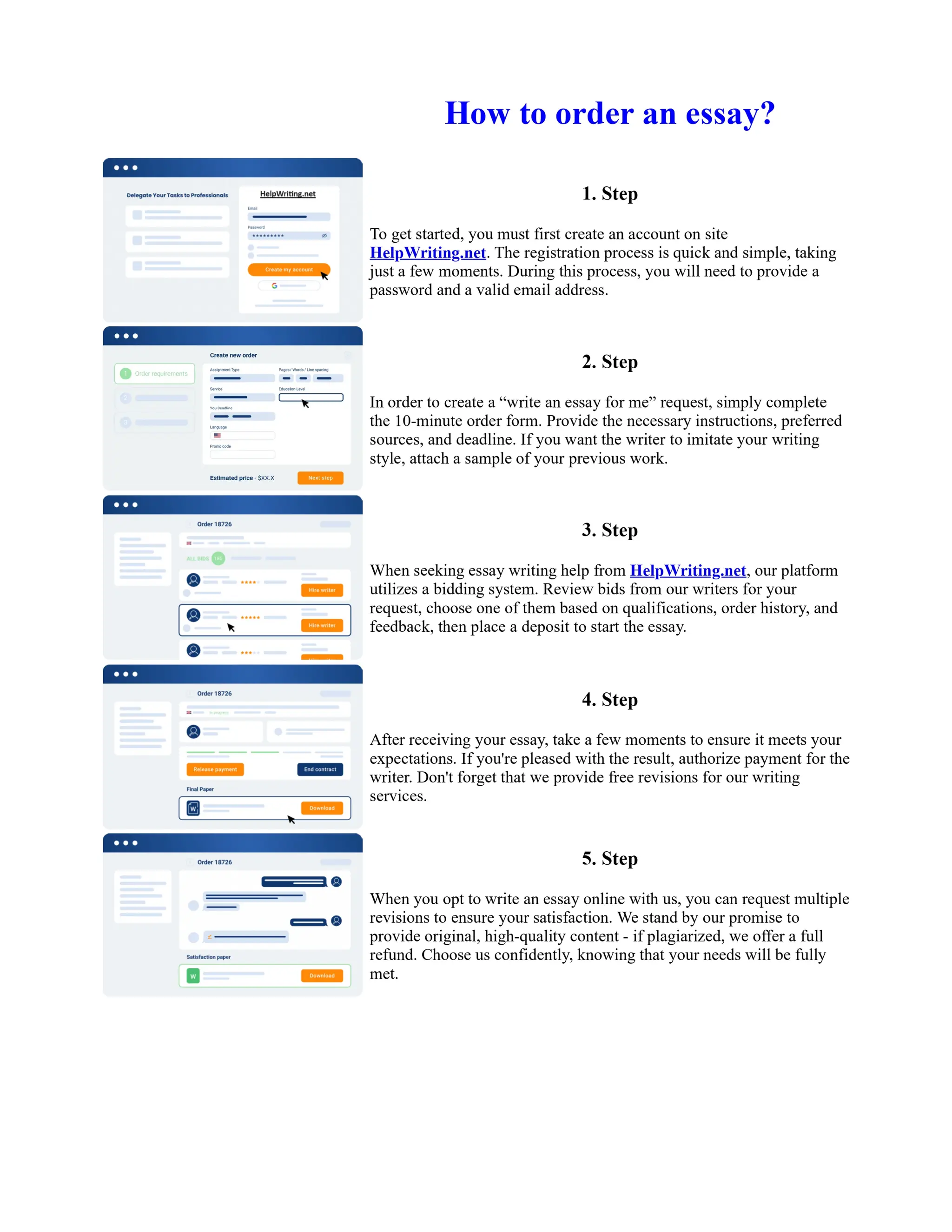
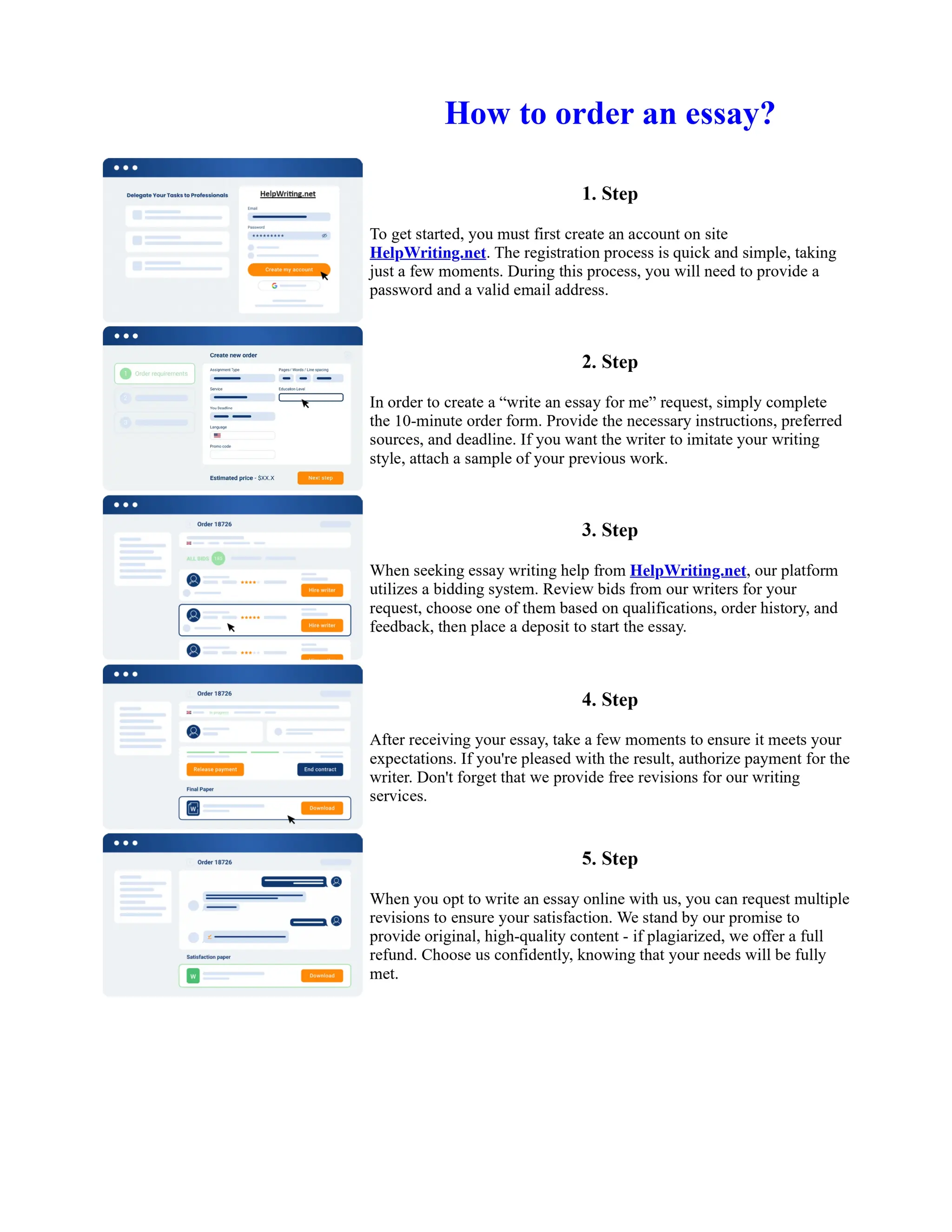
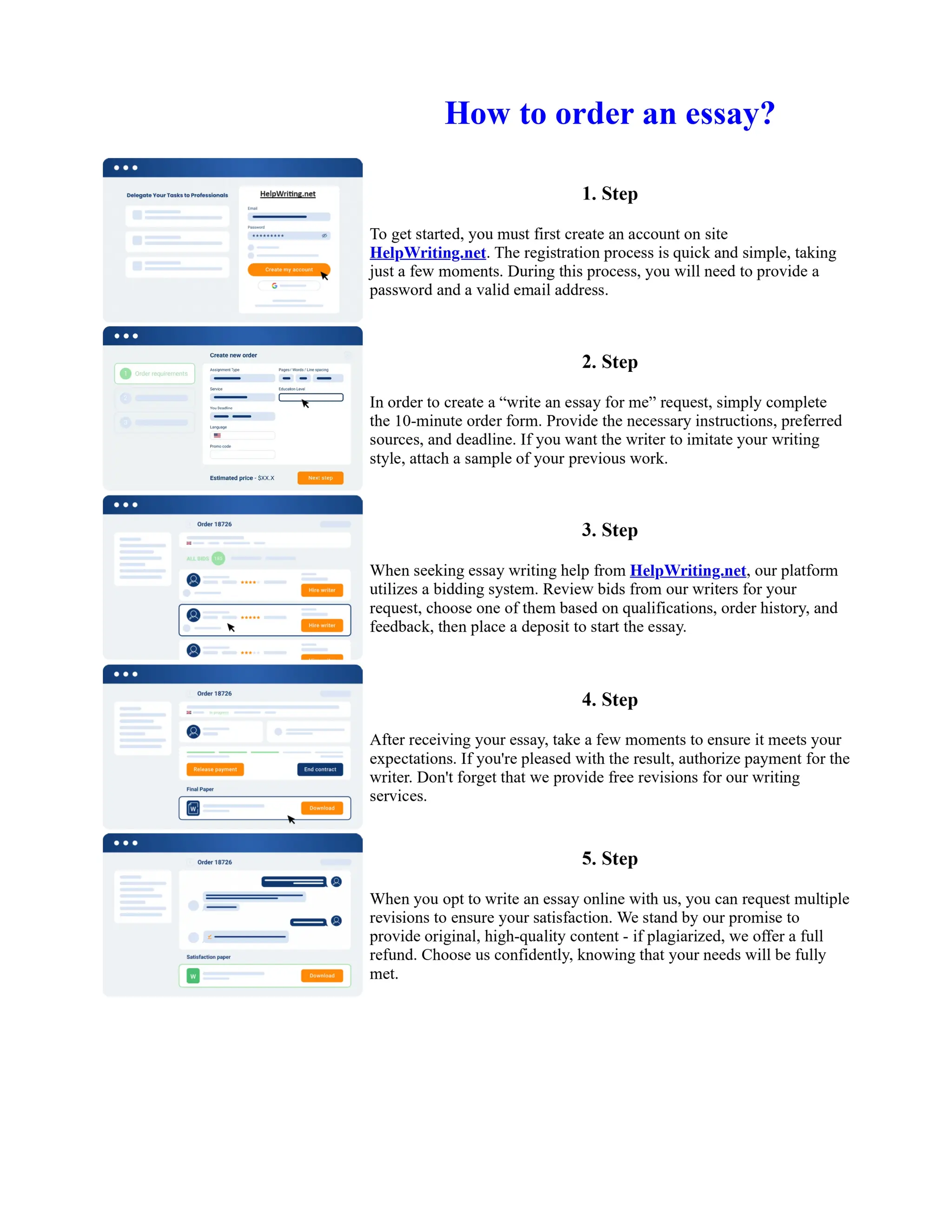
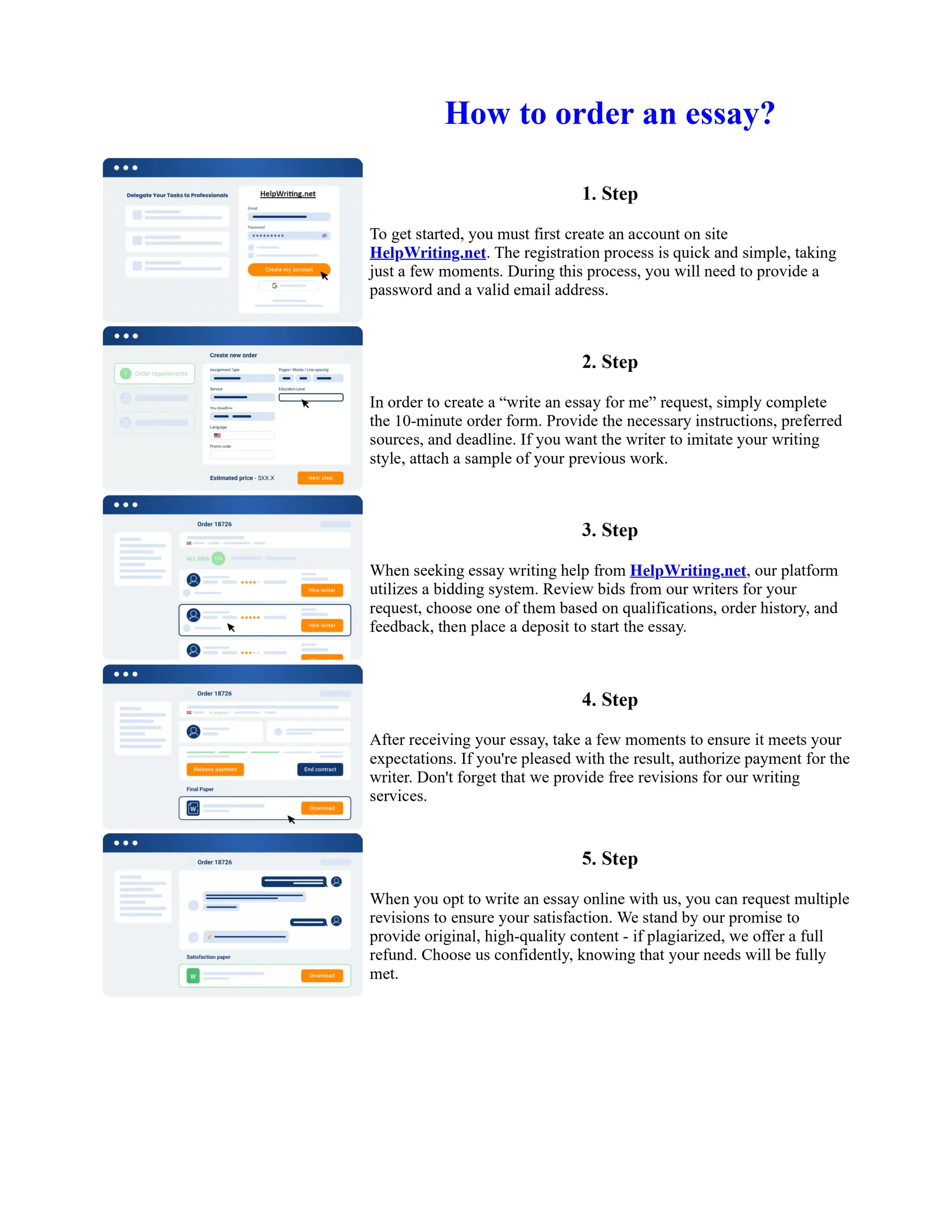
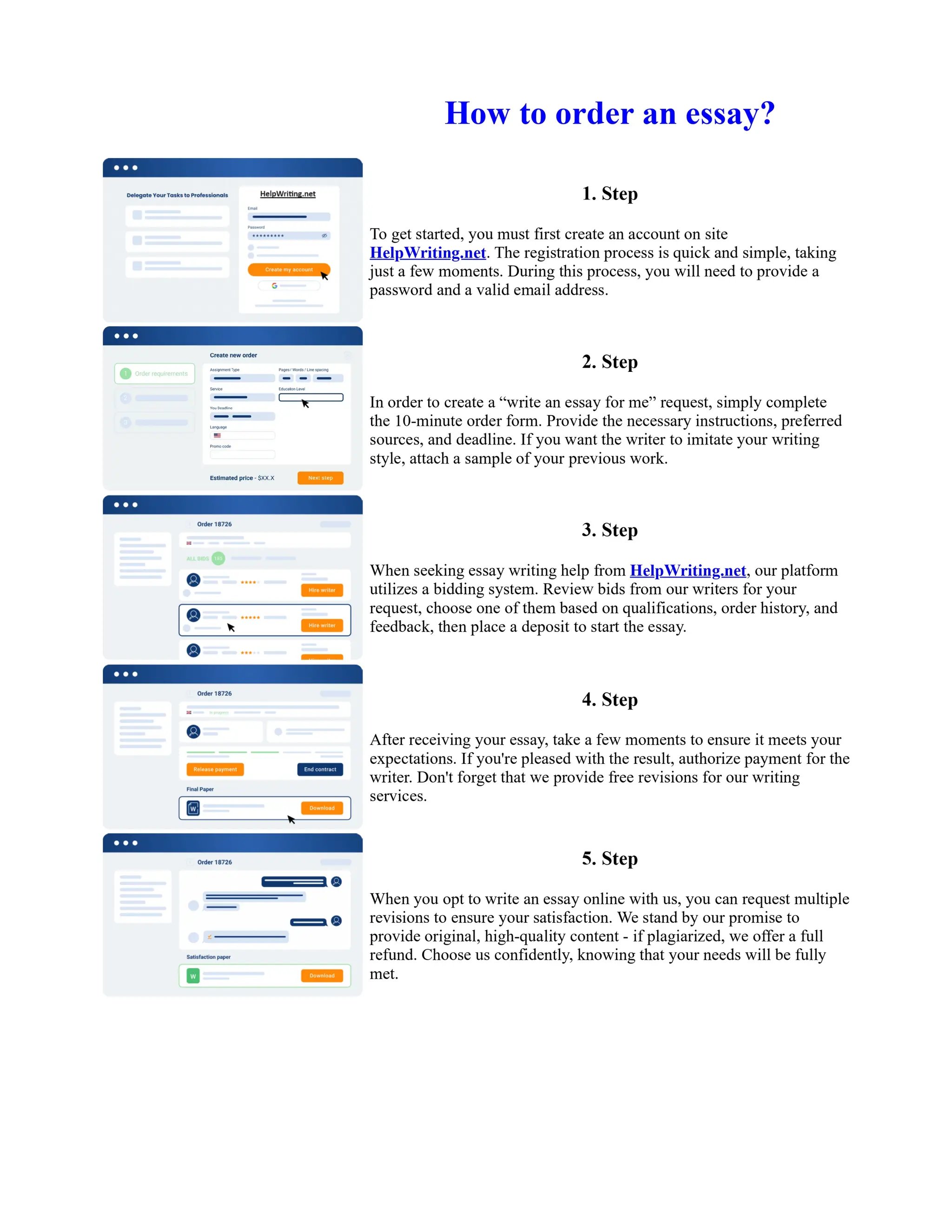
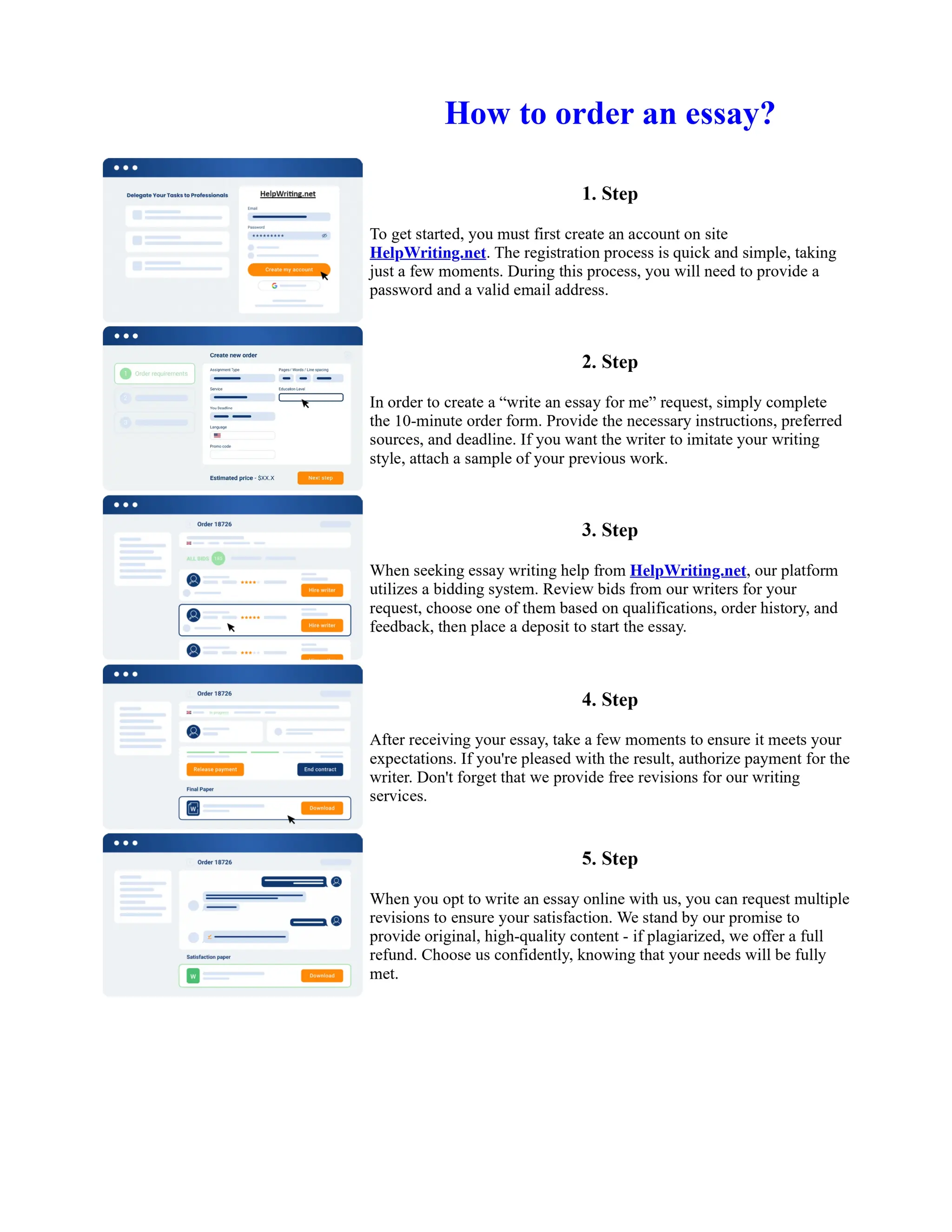
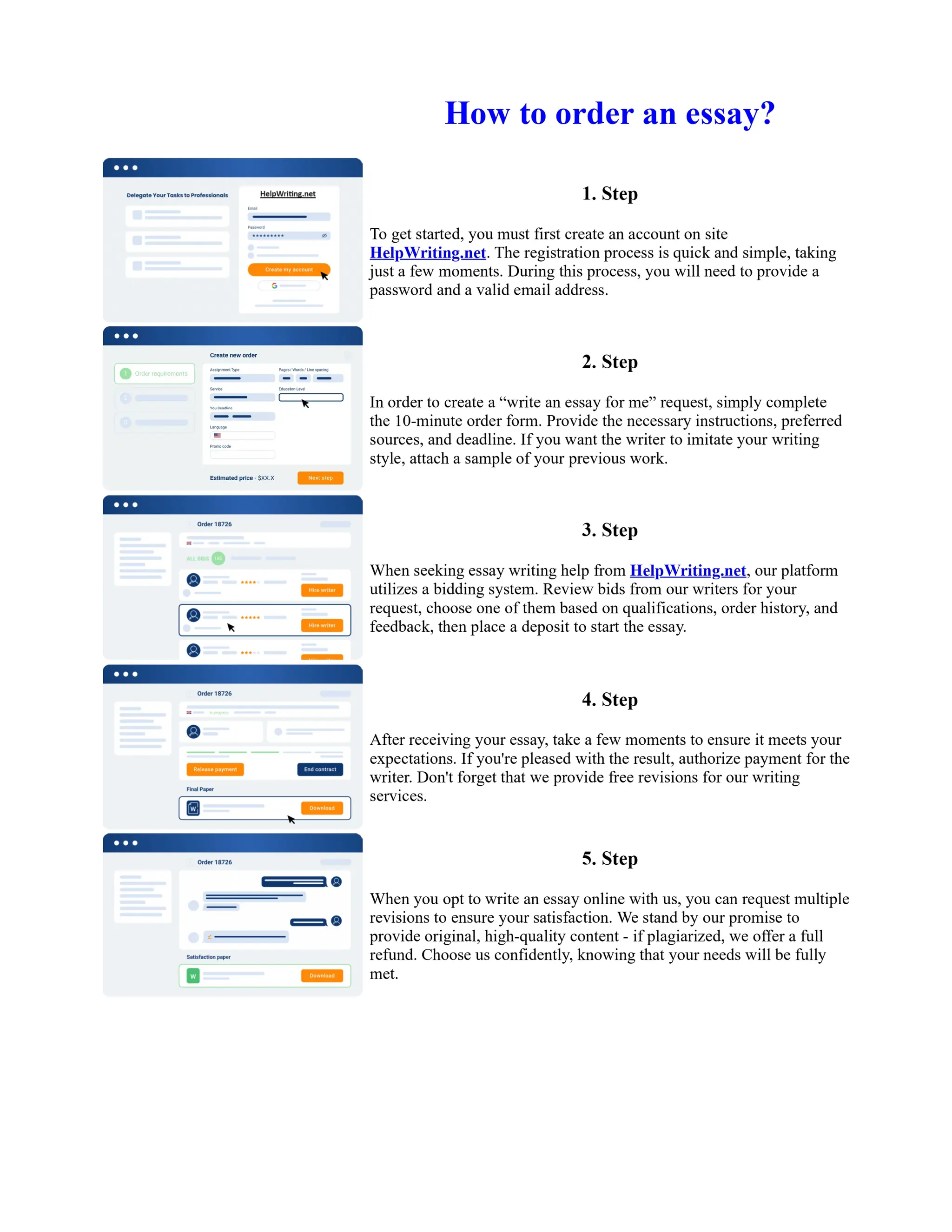
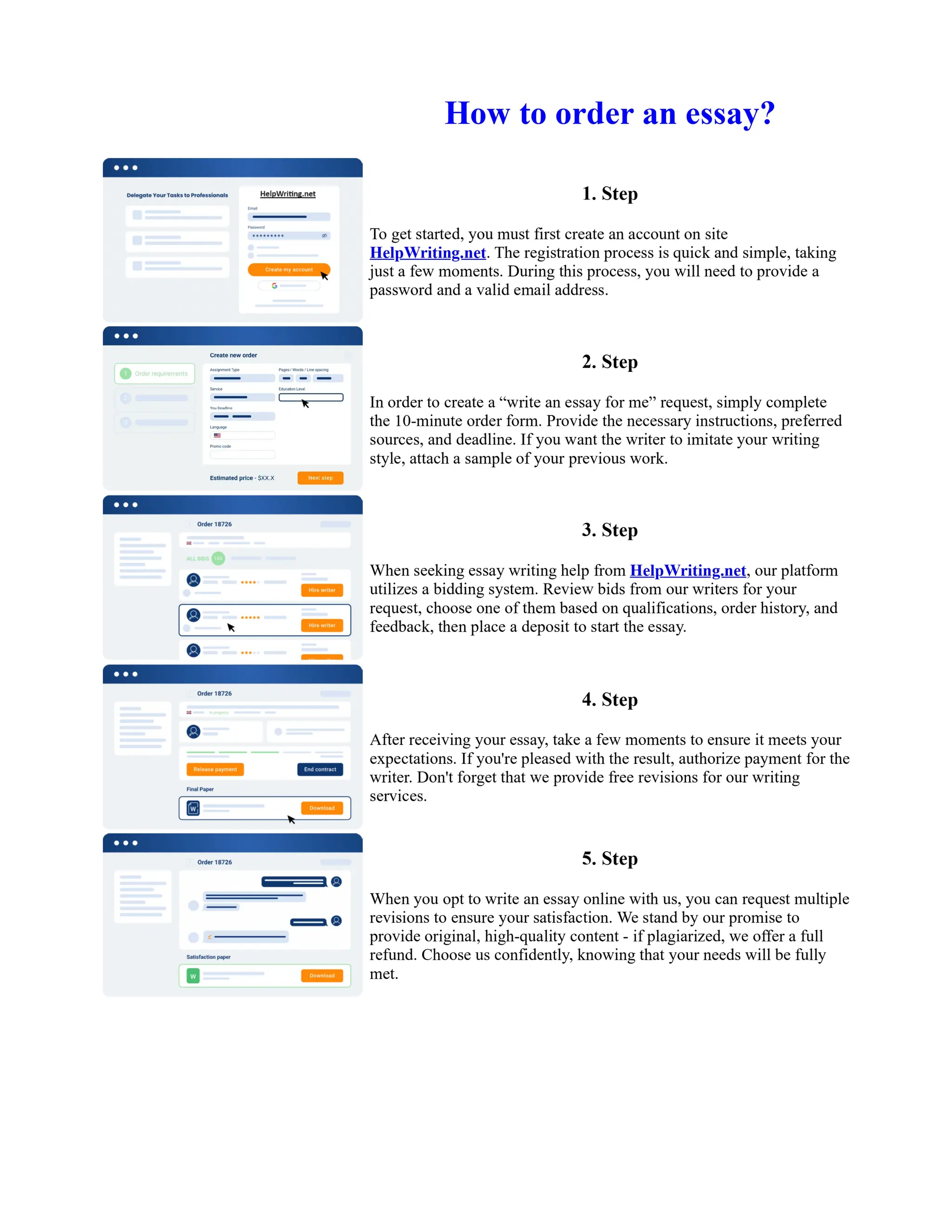
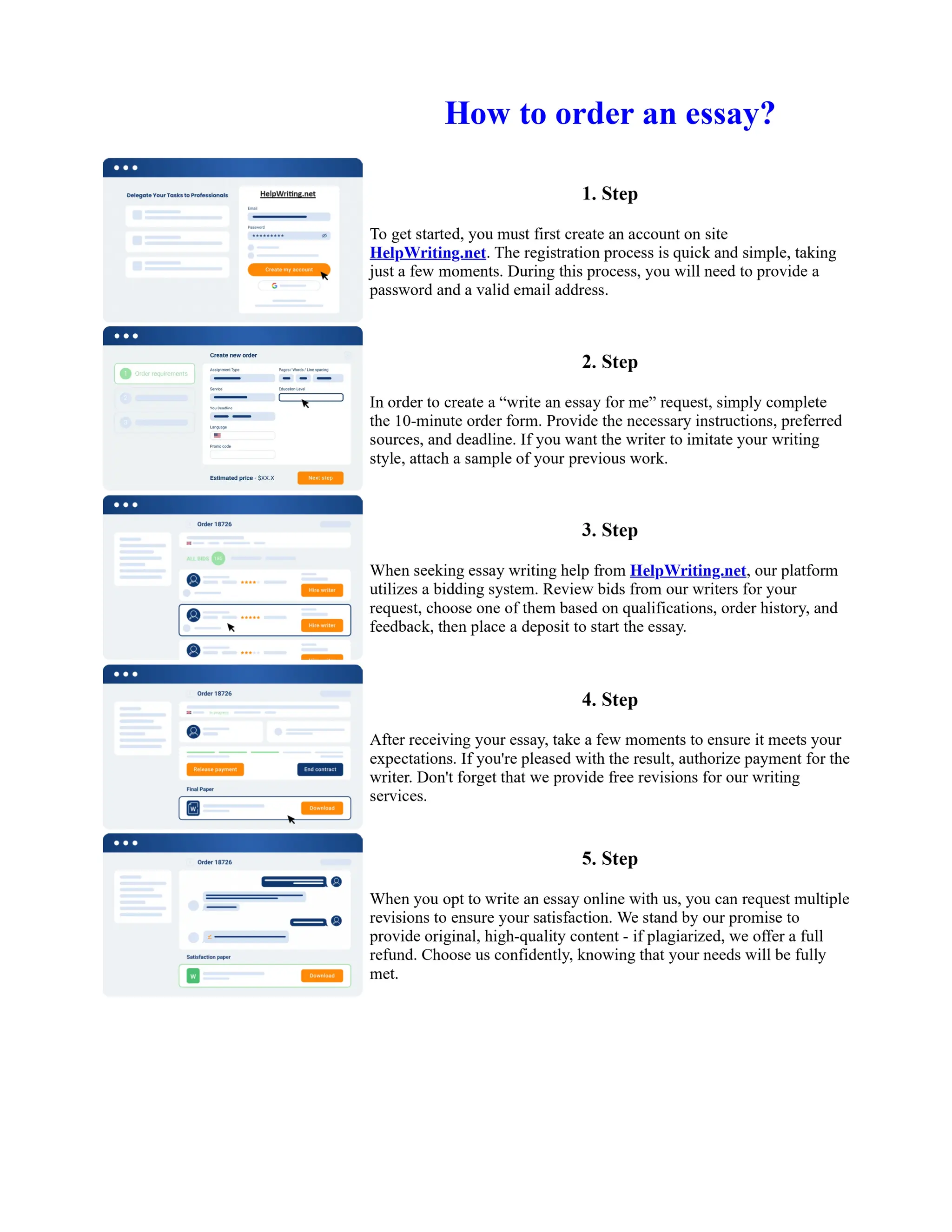
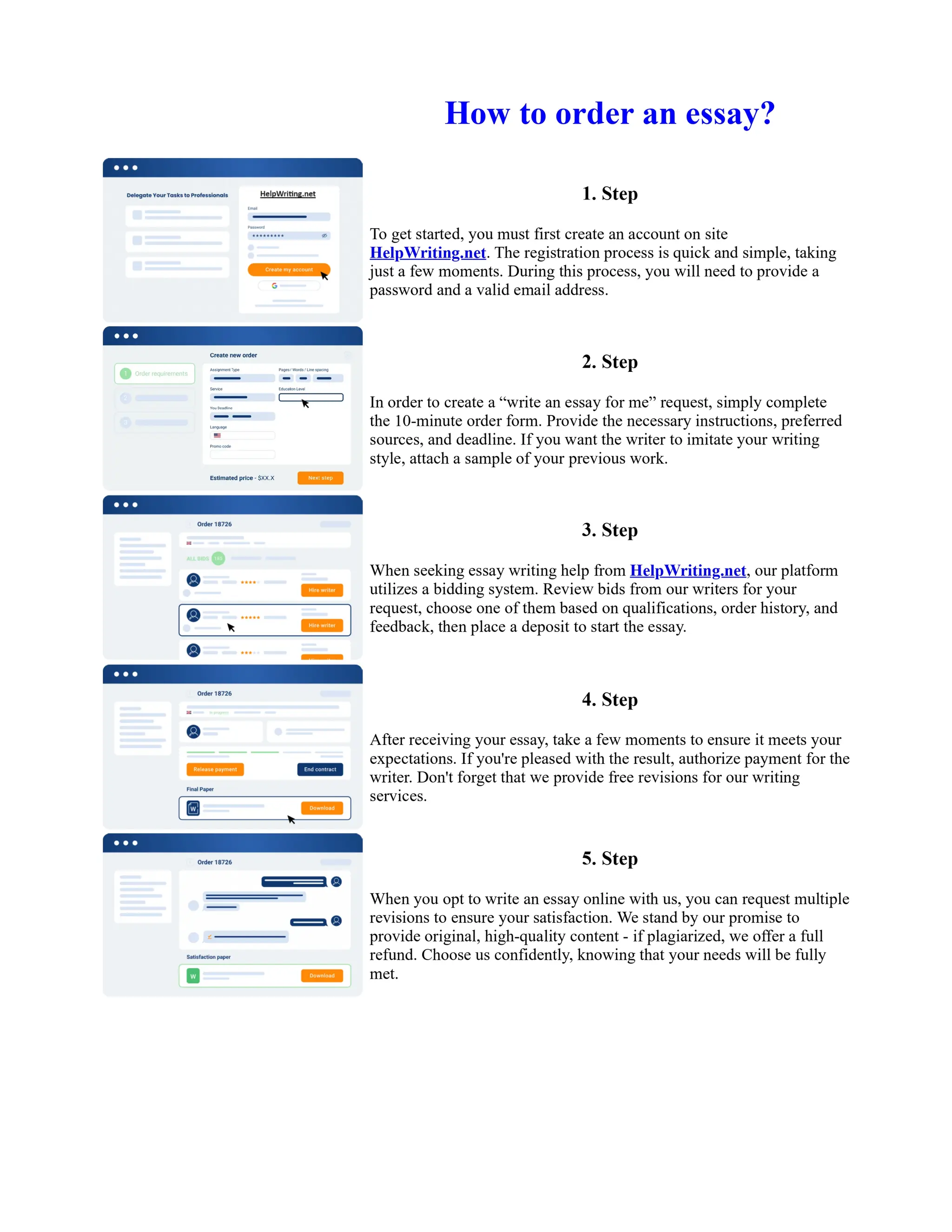
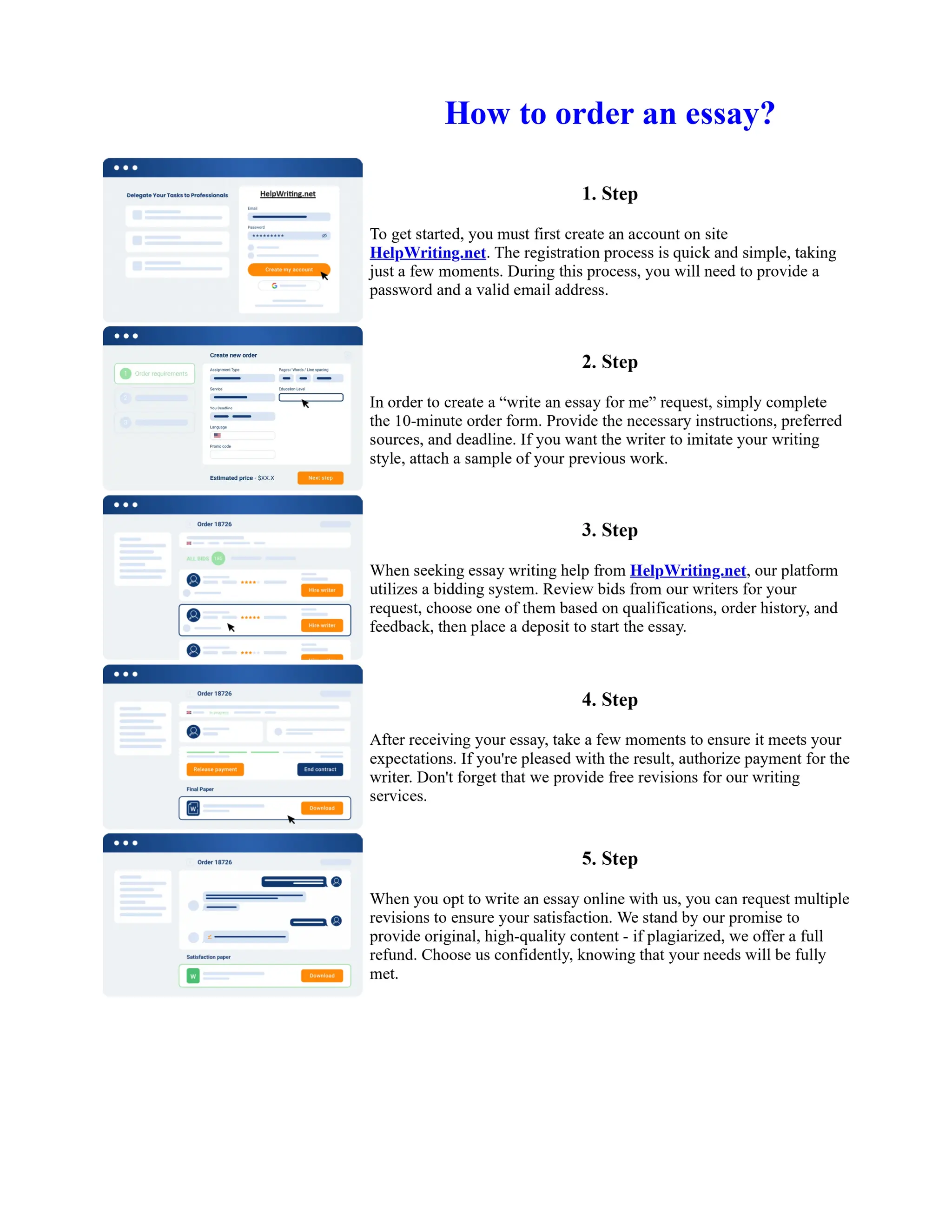
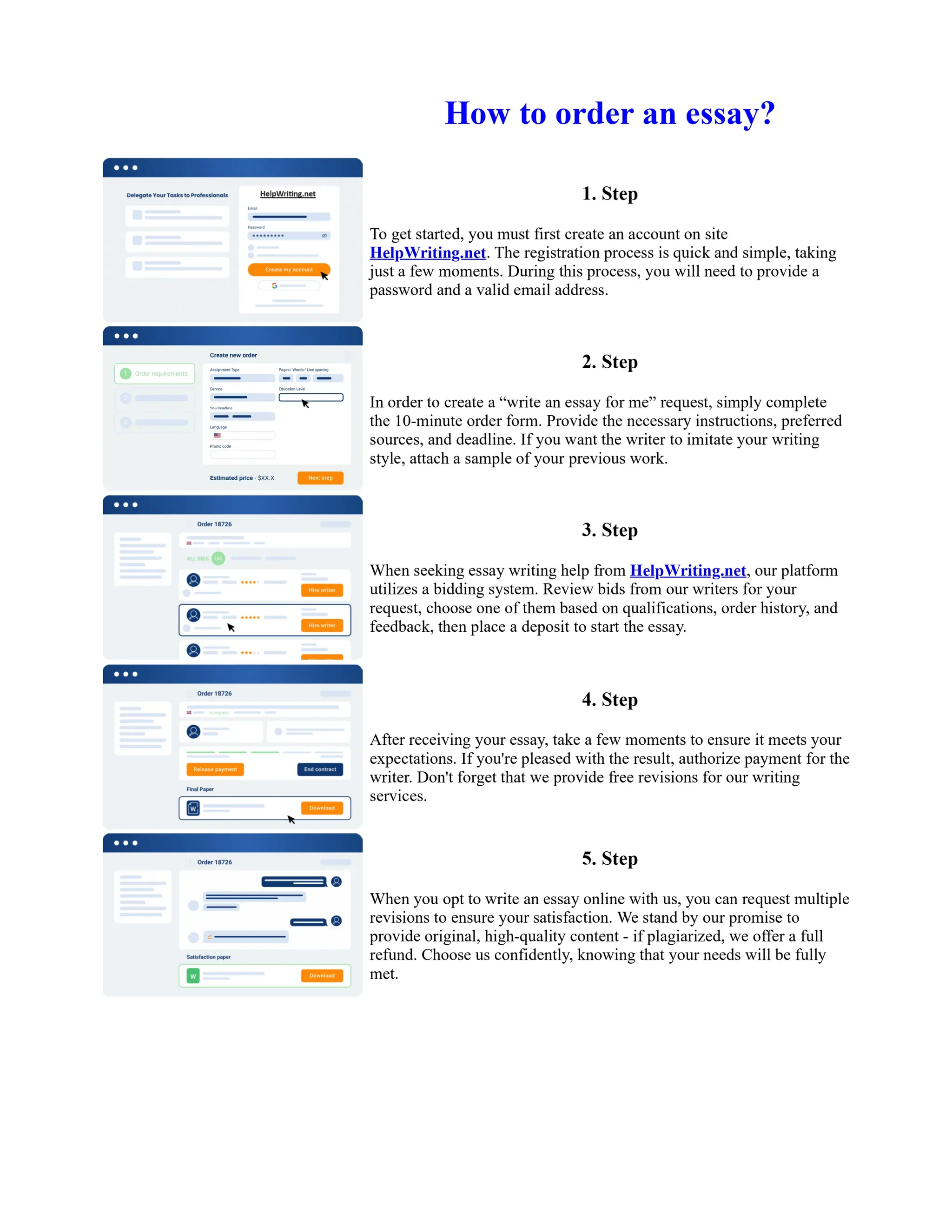
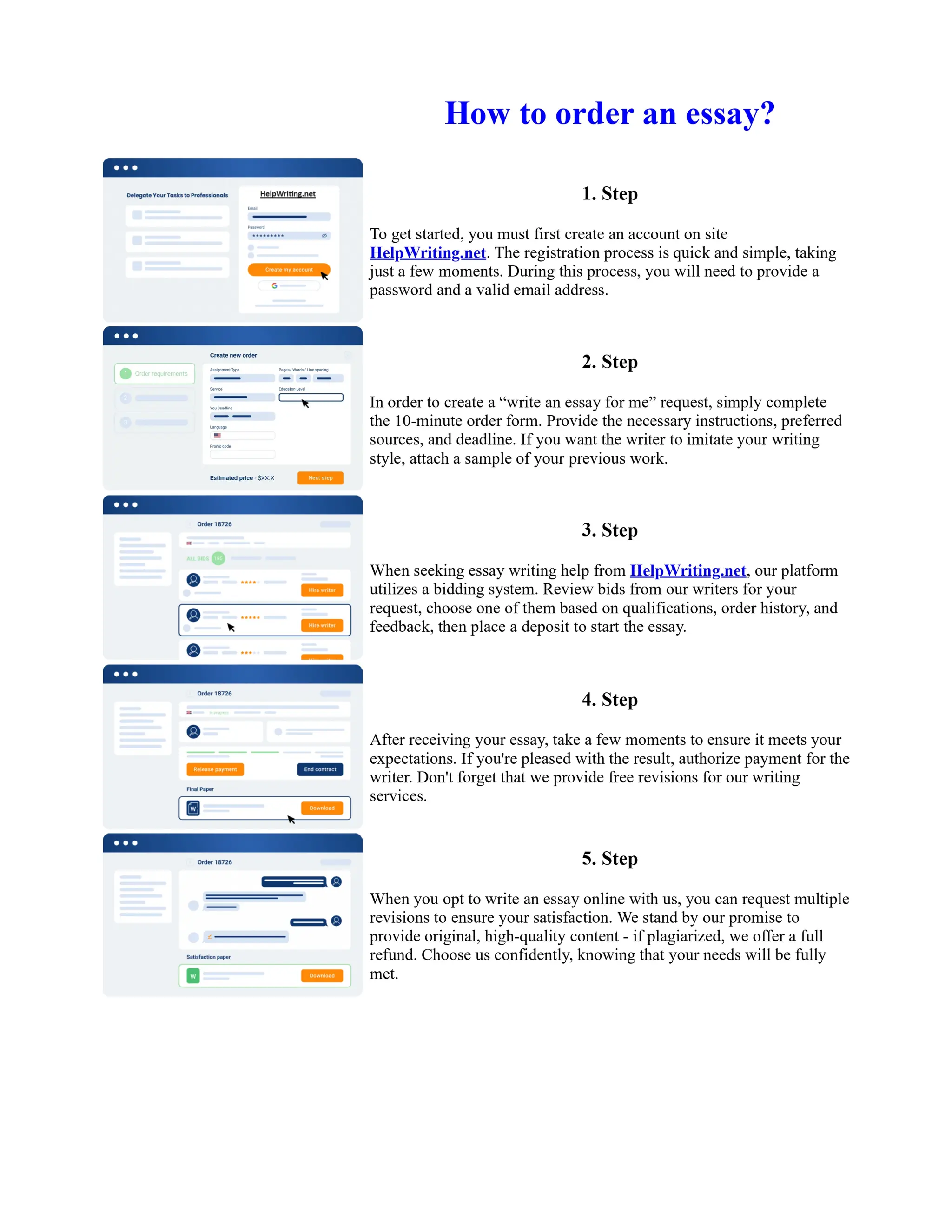
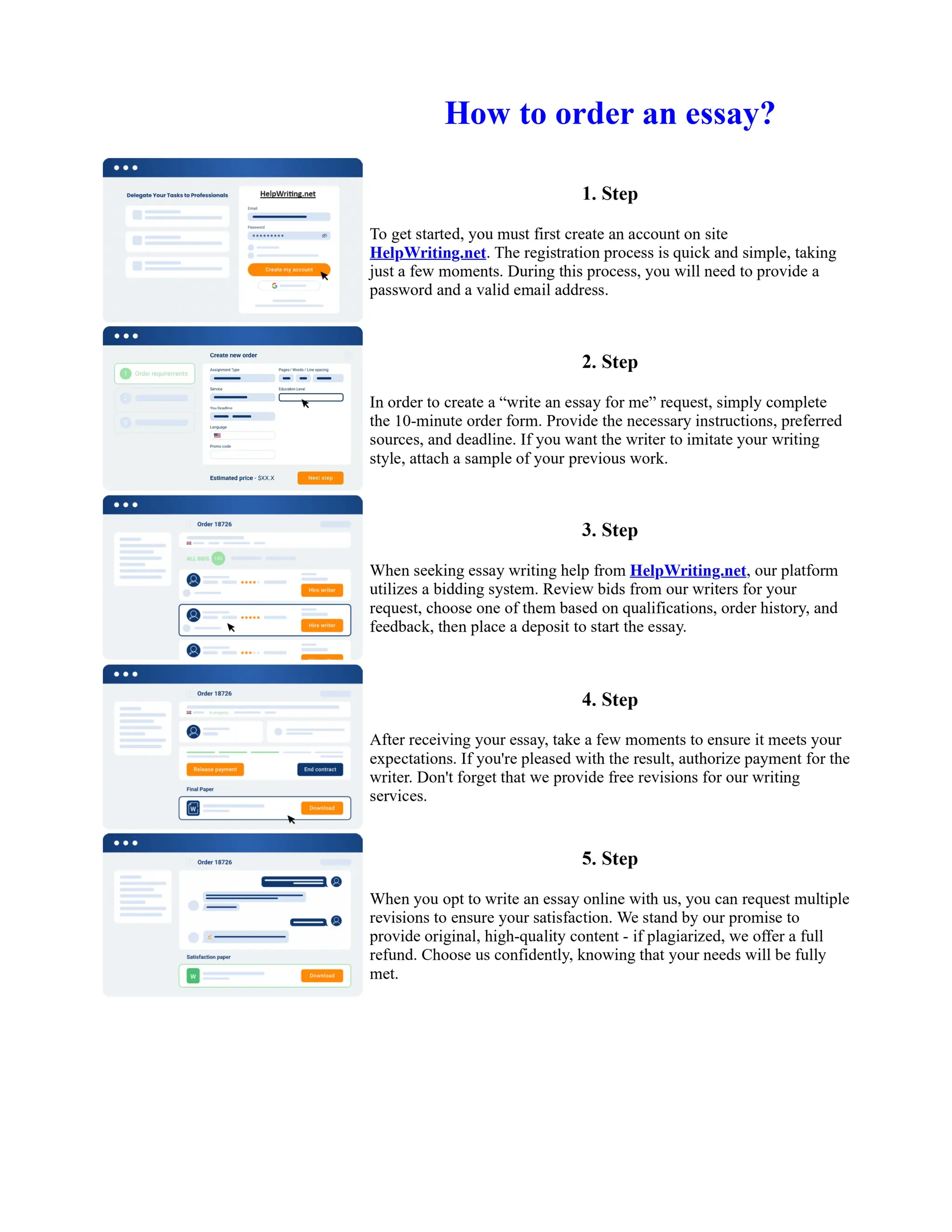
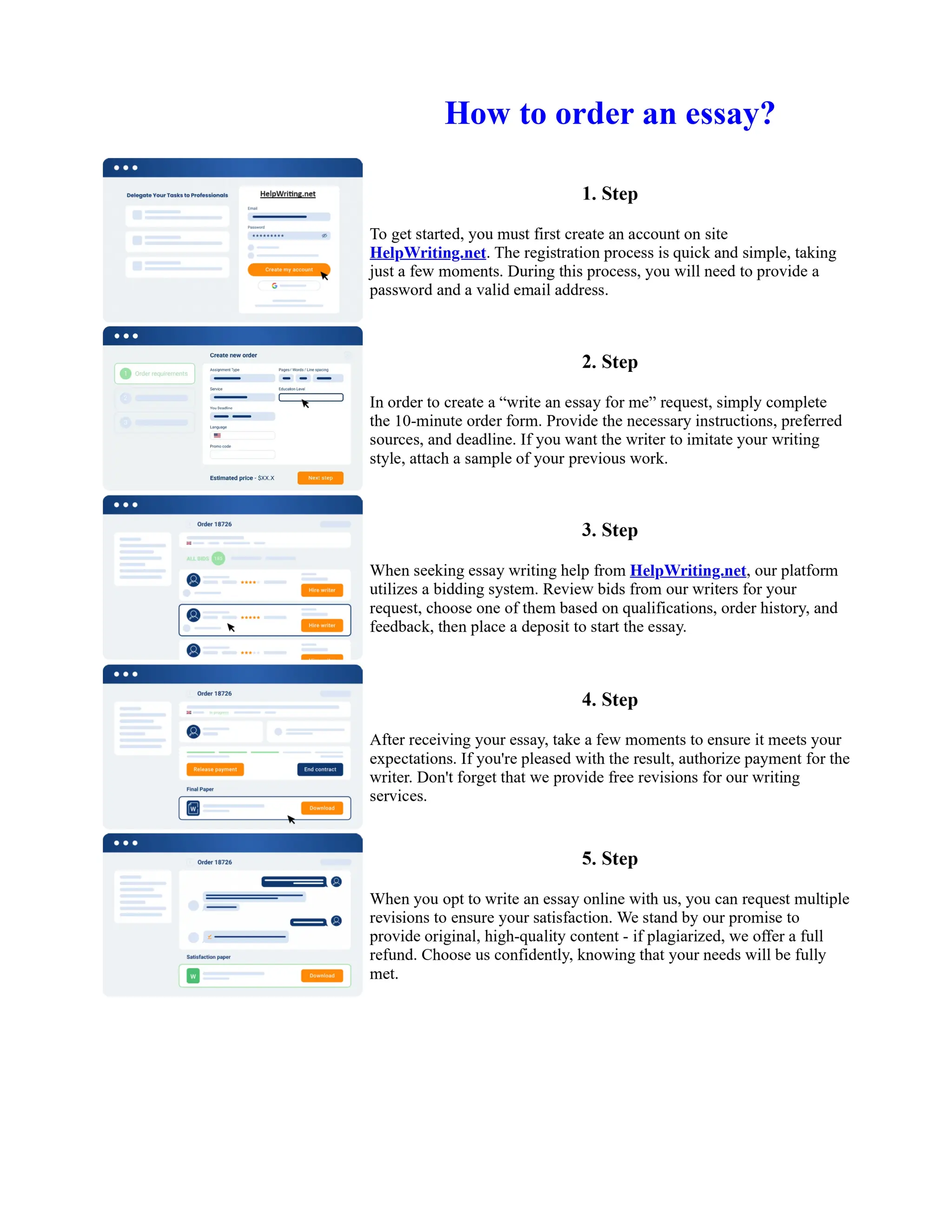
1. Isopentyl acetate was synthesized via an acid-catalyzed Fischer esterification of isopentyl alcohol and acetic acid. Sulfuric acid was used as the catalyst.
2. The reaction products were extracted with ether and the ether layer was dried over sodium sulfate. Distillation was then used to purify the product.
3. IR spectroscopy, gas chromatography, and comparison to an authentic isopentyl acetate sample were used to identify the final product as isopentyl acetate. The IR spectrum and GC results matched those of the
![Essay On Down Membrane
Synthesis and performance of cellulosic nonwoven forward osmosis membrane blend for water
desalination
Abstract
Recently, forward osmosis (FO) acquired a growing research area, because of its great potential for
low energy consumption and simple configuration of equipment used. Accordingly, FO was
investigated in a broad range of applications, comprising seawater and brackish water desalination,
wastewater treatment, food processing and power generation. The development of FO process can
be governed by two major factors; the development of high–performing draw solutions and highly
efficient FO membrane. It is the fact to say that, the core of the advancement in the FO process is
dedicated to membrane materials development to fabricate ... Show more content on Helpwriting.net
...
Fouling in FO membrane is physically reversible process that decreases the need for chemical
cleaning compared to reverse osmosis (RO) membrane [7, 8].
The development of FO process can be managed by two major factors:1) the development of high–
performing draw solutions and 2) using highly efficient FO membrane.
The desired FO membranes consist of the following characteristics: (1) an ultrathin semi–permeable
active layer with a high water flux and high solute rejection; (2) a thin supporting layer with high
porosity and low internal concentration polarization (ICP); (3) hydrophilic nature to enhance water
flux and reduce membrane fouling; and (4) sufficient mechanical strength and robust to sustain
backwash, cleaning and vibration in industrial operations [9, 10]. Recently, efforts were done to
develop new types of FO membranes with better properties. Based on the fabrication methods, the
newly developed membranes can be classified into two main kinds: cellulosic and thin film
composite (TFC).
Nowadays, the most common commercially FO membrane has been the asymmetric CTA
membrane. In spite of the CTA membranes have proper characteristics that including high chlorine
resistant and high hydrophobicity, they possess some drawbacks that hinder the future development.
The CTA membranes have limited stability to pH, temperature and microorganisms. Accordingly,
the feed and draw solutions must be maintain at pH between 4 and 6 and temperature below
... Get more on HelpWriting.net ...](https://image.slidesharecdn.com/essayondownmembrane-231207103924-5dc42ea9/75/Essay-On-Down-Membrane-1-2048.jpg)





















![PAE Synthesis Lab Report
2.2 Materials and Methods: Polyamino–ether was obtained from the collaborator Dr. Kaushal Rege
lab in Arizona state University. All other chemicals were obtained from Sigma–Aldrich (St. Louis,
MO). 2.2.1 Synthesis of the bioreducible modified–PAE: The obtained PAE polymer was
synthesized by reacting neomycin with glycidyl ether at molar ratio of 1:2 as reported in detail by
Rege et al. previously[14]. The modified bioreducible PAE(mPAE) was synthesized by a simple one
step reaction with Traut's reagent as described previously[15]. Briefly, 10g of PAE was dissolved in
1ml of ddH20 (Double distilled water) and was allowed to react with 1.85 g(5–times molar excess)
of 2–iminothiolane hydrochloride (IH) at room temperature for 15 h[16]. The product was dialyzed
using a dialysis membrane (MWCO = 1,000 Da) against 5 mM HCl for 24 h followed by dialysis
against 1 mM HCl solution for 24 h. The purified mPAE was lyophilized and stored at –20°C for
future use [17]. ... Show more content on Helpwriting.net ...
The mPAE was collected after extensive dialysis and freeze dried for future use. The modification of
amino groups (–NH2) is shown as representative
... Get more on HelpWriting.net ...](https://image.slidesharecdn.com/essayondownmembrane-231207103924-5dc42ea9/75/Essay-On-Down-Membrane-27-2048.jpg)

![Bismuth Synthesis Essay
Bismuth(III) metal is relatively inert, soft and malleable. The world production of bismuth(III) is
about 5000 tones per year [1]. Bismuth is seen as the least toxic heavy metal for human being and is
widely used in medical applications for its good antimicrobial properties. Bismuth(III) compounds
are used in cosmetics, pigments and a few pharmaceuticals, nearly 70% of the production being
used for this purpose. Bismuth(III) compounds have been used in medicines for more than 200 years
in variety of gastrointestinal disorders, because of their demulcent properties [2]. Presently, bismuth
is used in the cosmetics industry for the preparation of creams and hair dyes, while some of its
colloidal salts due to their antiseptic, astringent and diuretic ... Show more content on
Helpwriting.net ...
Synergistic mixture of N–n–octylaniline and trioctylamine used for extraction and separation of
bismuth(III) [8]. Literature survey revealed that 4–methyl–N–n–octylaniline [9] and N–n–
hexylaniline [10] were used for the solvent extraction of bismuth(III). The metal ion in the organic
phase was stripped and determined titrimetrically. It also suffers from multiple extraction of
bismuth(III) with 1622 hymine [11] in different solvents from nitric acid, mercury, thorium and
uranium interfered seriously. Many reports show that extraction of bismuth(III) is carried out by
using high molecular weight amines as liquid anion exchangers such as, 1622 hymine [11],
amberlite LA–1 [12], tri–iso–octylamine [12], aliquot 336 [12], tri–n–octylamine (TOA) [13–15],
tris (2–ethylhexyl) amine [16] and C10C12 primary amines [17]. Other authors have proposed the
extraction of Bismuth from acid or highly acidic solutions of HCl, HBr, HNO3 and/or H2SO4 using
Cyanex 925, Cyanex 921, 2–bromoalkanoic acid and Cyanex 302 as extractant [18–21].
Bismuth(III) in alloy samples was determined extractive spectrophotometrically using 1–amino–
4,4,6–trimethyl–(1H,4H) pyrimidine–2–thiol [22]. Spectrophotometric and atomic absorption
spectrometric methods have been developed for the determination of fluoroquinolone antibacterials
by ion–pair complex formation with bismuth(III) tetraiodide [23]. A study on bismuth(III) extraction
and its separation from other elements was carried out with triphenylarsineoxide [24]. Cyanex–925
in xylene was used for extraction separation of bismuth(III) from aqueous solution [25]. Bis–(2,4,4–
trimethylpentyl) monothiophosphinic acid (cyanex 302) [26] is predominant organophosphorus
extractant used for the extraction of bismuth(III). Bismuth was extracted quantitatively with 0.05M
18–crown–6 in methylene chloride
... Get more on HelpWriting.net ...](https://image.slidesharecdn.com/essayondownmembrane-231207103924-5dc42ea9/75/Essay-On-Down-Membrane-31-2048.jpg)























![Determining The Ksp Value For Silver Acetate Essay
Determining the Ksp value for silver acetate.
Introduction:
An equilibrium constant for a solid substance that is dissolving in an aqueous solution is represented
by the solubility product constant, where the concentrations of different ions from the solid
substance (compound) are used to calculate the solubility product constant. If the solubility product
constant vale is high then the substance is very soluble in an aqueous solution. The solubility
product constant is denoted by Ksp, and for a common reaction: aA(s) ⇌ cC(aq)+dD(aq)T
The Ksp= [C(aq)]c[D(aq)]d
The concentration of the solid (aA) doesn't contribute to the equilibrium constant because their
concentration doesn't change the expression of the equilibrium constant. Any change in their
concentration isn't that important as the ions and therefore they are taken out of the expression,
hence, the solubility product constant represents the amount of solid that can be dissolved in a
solution.
One important effect on the solubility ... Show more content on Helpwriting.net ...
The solution was swirled to induce precipitation, the swirling was for 30 minutes. The solution was
filtered through a dry filter paper and a dry funnel into a dry 250 ml beaker. The temperature of the
filtrate was then measured and recorded. A clean, dry burette was assembled and filled with the
potassium thiocyanate solution, the burette reading was recorded, then into a clean, 50 ml flask, 5.00
ml of the filtered silver acetate solution was pipetted. 30 drops of the indicator (saturated ferric alum
in 1.0 N HNO3), a few drop of the thiocyanate solution was added, then continued to be added
dropwise, with swirling after each addition, until one drop gives a permanent light orange colour.
The whole process of titrating silver acetate with potassium thiocyanate was repeated two more
times with batches of 5.00 ml of the saturated silver acetate
... Get more on HelpWriting.net ...](https://image.slidesharecdn.com/essayondownmembrane-231207103924-5dc42ea9/75/Essay-On-Down-Membrane-60-2048.jpg)



![Chemistry Of Zinc Acetate Dihydrate
The chemicals used in the experiment were analytical reagent grade and were used without further
purification. Zinc acetate monohydrate [Zn(CH3COO)2 .H2O] Merck > 98%, and sodium
hydroxide (99.5%, NaOH) pellets were purchased from R &M chemicals (UK). Indium nitrate
trihydrate, distilled water was ethanol used throughout the experiments, PEG 20000, Triton X–100.
For device fabrication materials were procured from Solaronix. In a typical synthesis process, the
reaction solution was prepared by mixing 6g of Zinc acetate dihydrate in 70:30 ratio of
ethanol/water. The ZnO precursor containing In–dopant was prepared with 0.0, 1.0, 3.0 and 5.0 mole
% of indium. A thick white gel was formed after the addition of NaOH into the reaction solution, the
mixture was kept under magnetic stirring for 24h to provide perfect growth, the resulting white gel
was dried and finally calcined at 200ºC for 5h. The as–prepared pristine ZnO:P and ZnO:In3+
samples were characterized via X–ray diffraction (XRD) using Cu–Kα radiation (λ= 1.54056 Å) in
the 2θ range of 20°–80° [Bruker Advanced–D8 powder X–ray diffractometer]. The scanning speed
and steps were 2°/ minute and 0.02° of 2θ respectively. The XRD data were analyzed by Rietveld
refinement technique using FULLPROF program to confirm the phase formation as well as to obtain
the lattice parameters, space group and crystal system [2]. The microstructures and crystal structures
of the nanoparticles were obtained using Transmission Electron
... Get more on HelpWriting.net ...](https://image.slidesharecdn.com/essayondownmembrane-231207103924-5dc42ea9/75/Essay-On-Down-Membrane-65-2048.jpg)











![Silver Nanoparticle Synthesis Lab Report
Materials Used. For silver nanoparticle synthesis, silver acetate (AgAc–166.91g/mol) was procured
from Sigma Aldrich and oleylamine from. Thiol terminated polystyrene (PS–SH) Mn–1100, Mw–
1230, PDI–1.12 was procured from Polymer Source Inc. Canada. The polymer for micelles
Polystyrene–b–4vinylpyridine (PS–b–4VP) Mn(PS)=18,500; Mn(P4VP)=40,500; PDI–1.10 was
procured from Polymer Source Inc. Canada. The Si sol precursor tetra ethyl orthosilicate (TEOS)
was procured from Sigma Aldrich. Sodium borohydride and p–nitrophenol was procured from
Sigma Aldrich (99.9%) and Across Organics (95%) respectively. Organic solvents including Toluene
and Methanol were procured from Acros Organics and Chloroform from Fischer chemicals and
Ethanol from VWR PROLABO was of analytical grade and used as received. Millipore water (18.2
ohm cm at 25 degree C, cleaned with Pure Lab Plus ® ultra purification system) was used as solvent
throughout the experiment and used as received. Synthesis of Polystyrene Capped Silver
Nanoparticles. Silver nanoparticles were synthesised using a simple method described by Hiramatsu
[Hir01]. For the ... Show more content on Helpwriting.net ...
10mg of PS–SH was dissolved in chloroform in 1:1 proportion with the nanoparticles and added to
silver nanoparticle/chloroform dispersion drop by drop under stirring. 5ml more chloroform was
added to the AgNP/PS mixture and was stirred for 24hours. Thereafter Ag/PS mixture was
reprecipitated in 10ml of Methanol and centrifuged at 13500 RPM for 10 minutes. To remove the
rest of OlAm and excess of PS–SH, repeated (6–8 times) centrifugation/redispersion steps were
performed by adding chloroform as a solvent (ca. 5 ml) and methanol as flocculating agent (ca. 10
ml for each step). Final product was collected and dried under argon to give a fine metallic powder
of ca. 11mg
... Get more on HelpWriting.net ...](https://image.slidesharecdn.com/essayondownmembrane-231207103924-5dc42ea9/75/Essay-On-Down-Membrane-80-2048.jpg)




