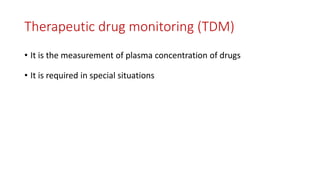
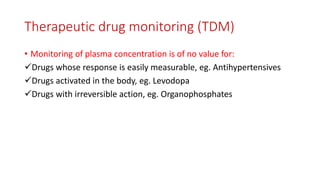
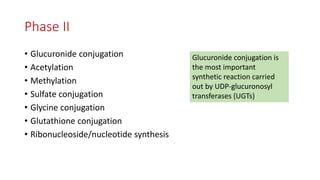
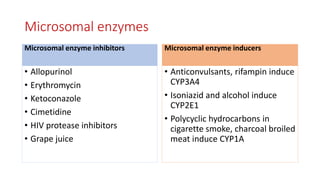
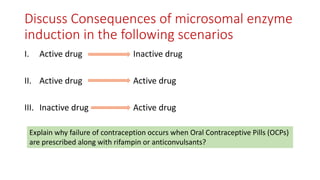
The document discusses drug metabolism and biotransformation. It notes that the liver is the primary site of biotransformation, which converts non-polar compounds to polar ones for excretion. Biotransformation can inactivate drugs, produce active metabolites, or activate inactive prodrugs. It occurs in two phases: phase I involves oxidation, reduction, and hydrolysis; phase II involves conjugation. Cytochrome P450 enzymes, especially CYP3A4, are responsible for many phase I reactions. Drug metabolism determines a drug's half-life, clearance, and affects dosing. Therapeutic drug monitoring measures drug concentrations to optimize dosing for drugs with a narrow therapeutic index.















![Kinetics of elimination
• First order kinetics:
V = rate of reaction = Vmax [C] / Km
Km = Michaelis constant
• Zero order kinetics:
V = rate of reaction = Vmax [C] /[C]
= Vmax](https://image.slidesharecdn.com/pharmacokineticprinciples-2-200815203658/85/Pharmacokinetic-principles-2-19-320.jpg)