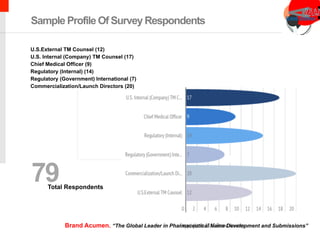
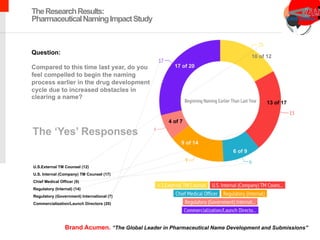
The document summarizes the key findings of a study on pharmaceutical naming and trademark trends conducted by Brand Acumen in 2015. The study found that drug companies are developing trademarks earlier in the drug development process, with 91% developing generic names and 87% developing trademarks before or during Phase II trials. The study recommends seeking trademarks as early as Phase I to improve chances of clearance. Additionally, emerging business models are driving earlier proprietary name development to enhance valuations and access to funding.