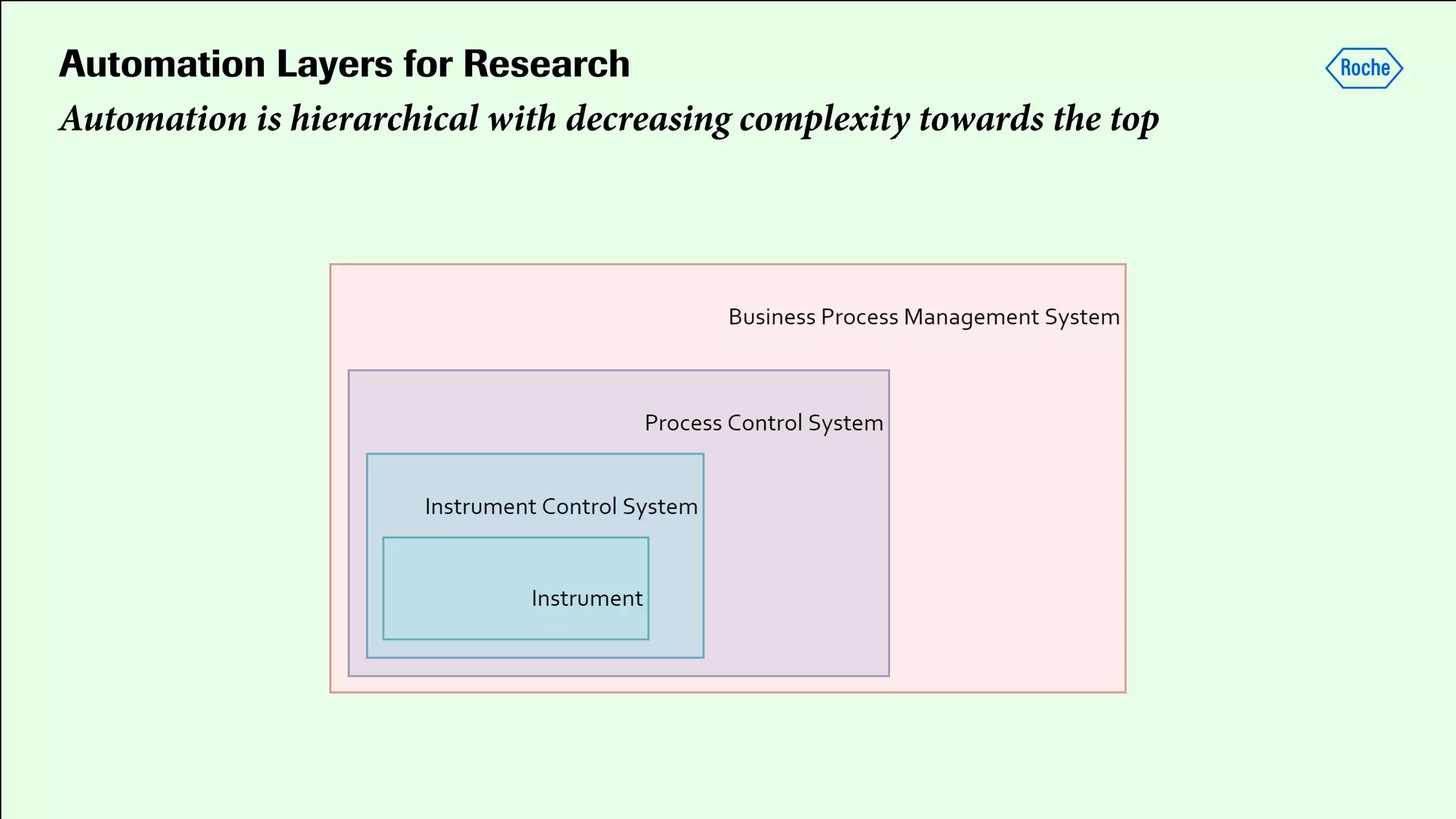
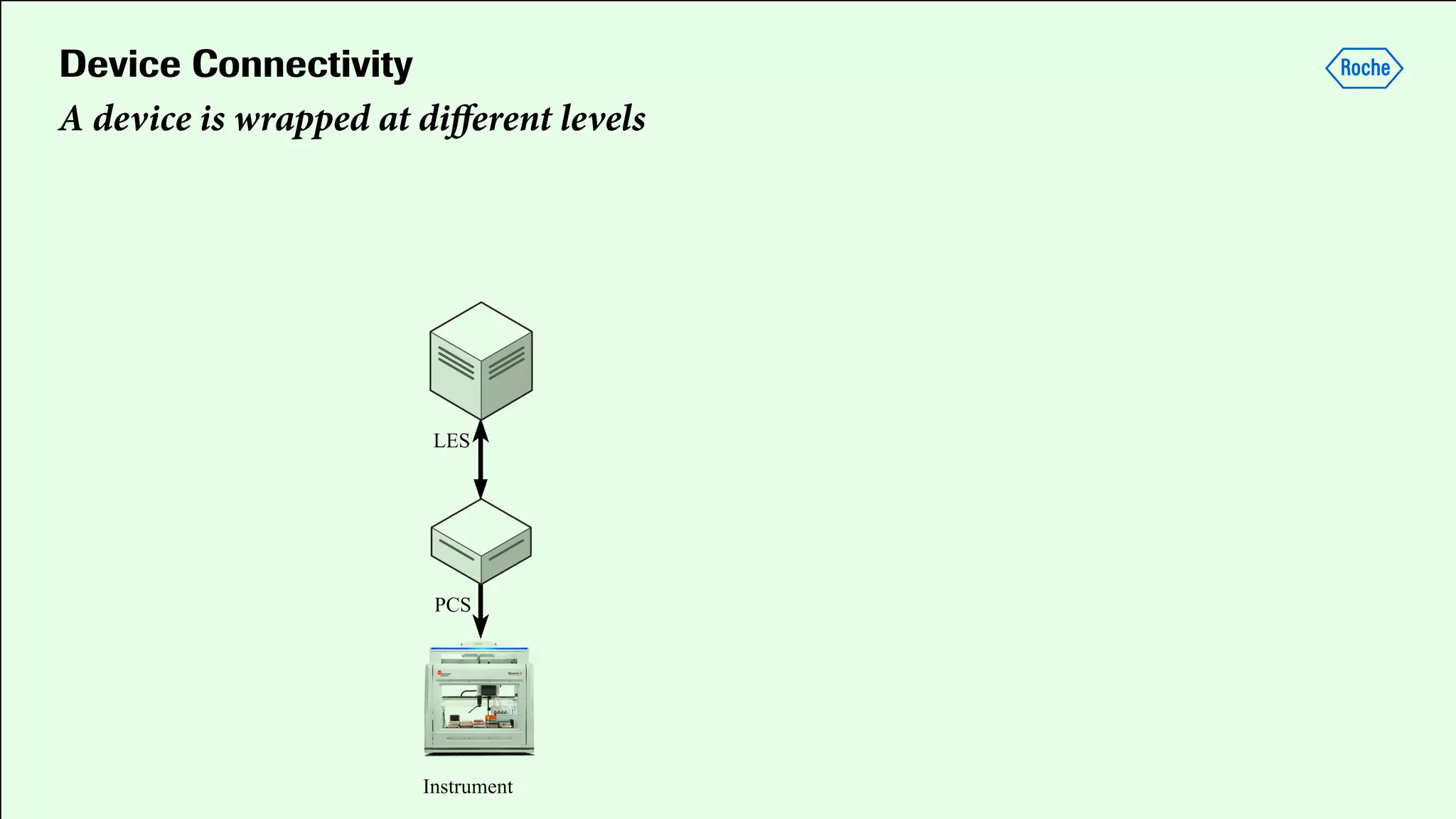
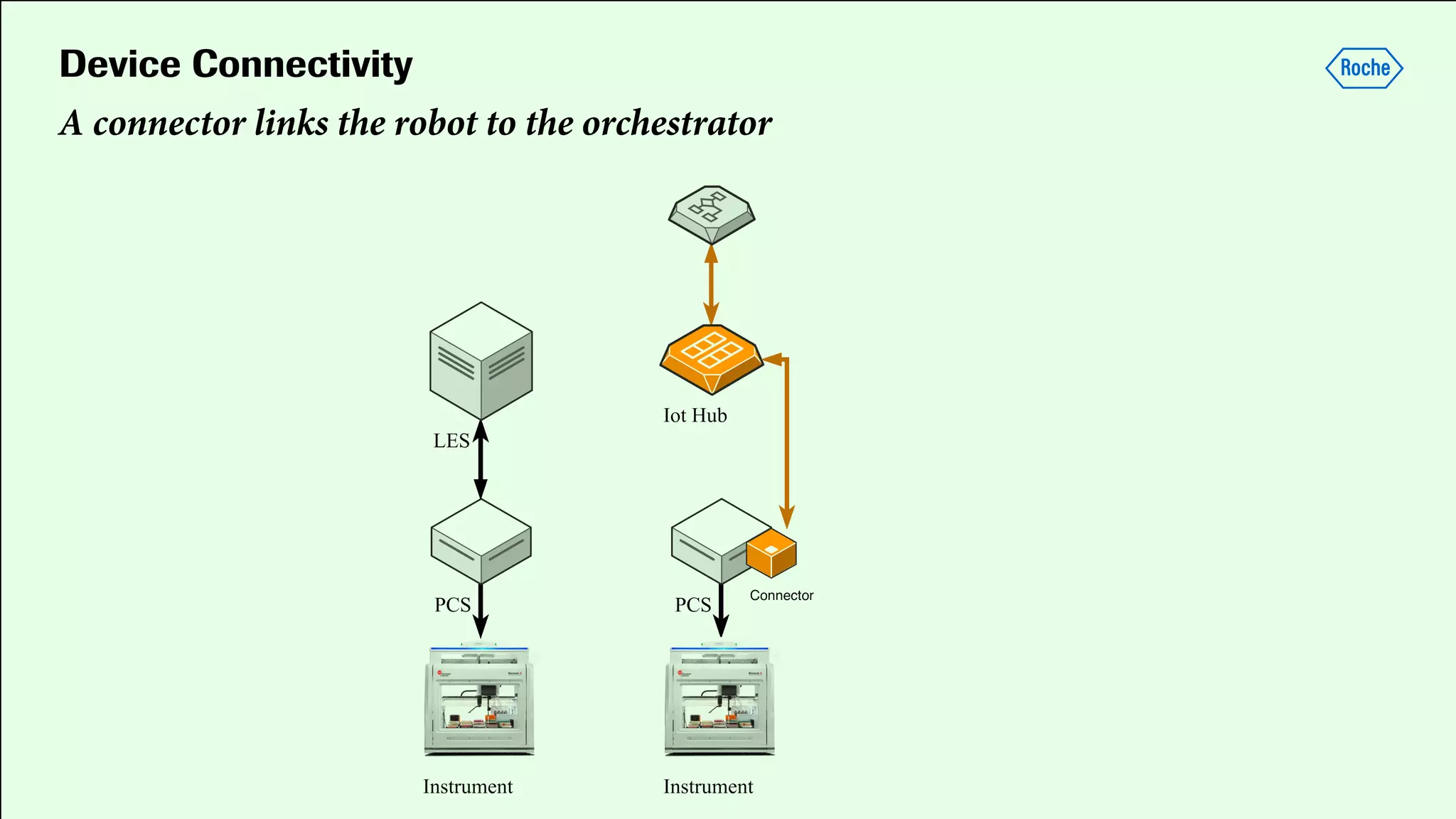
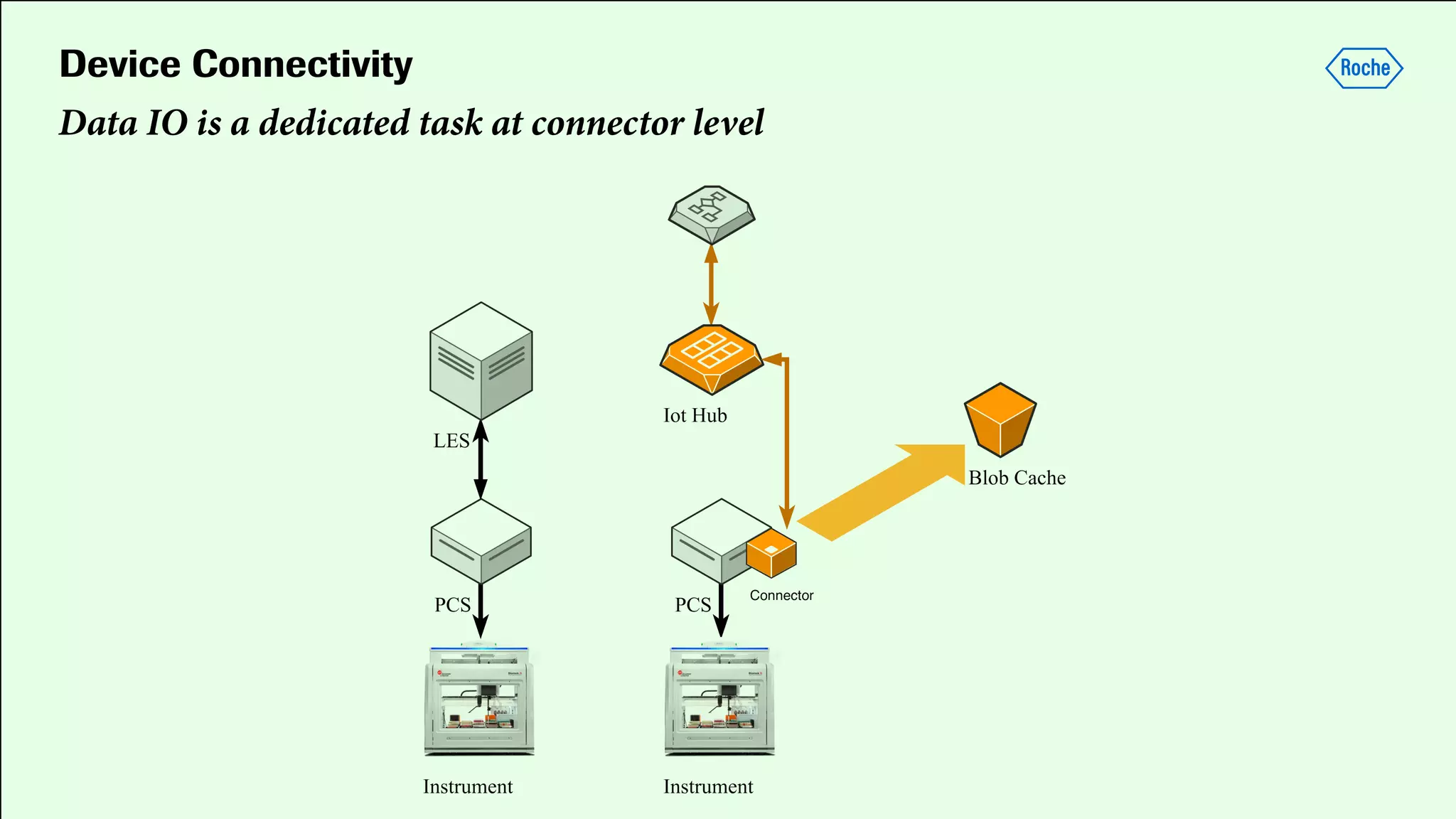
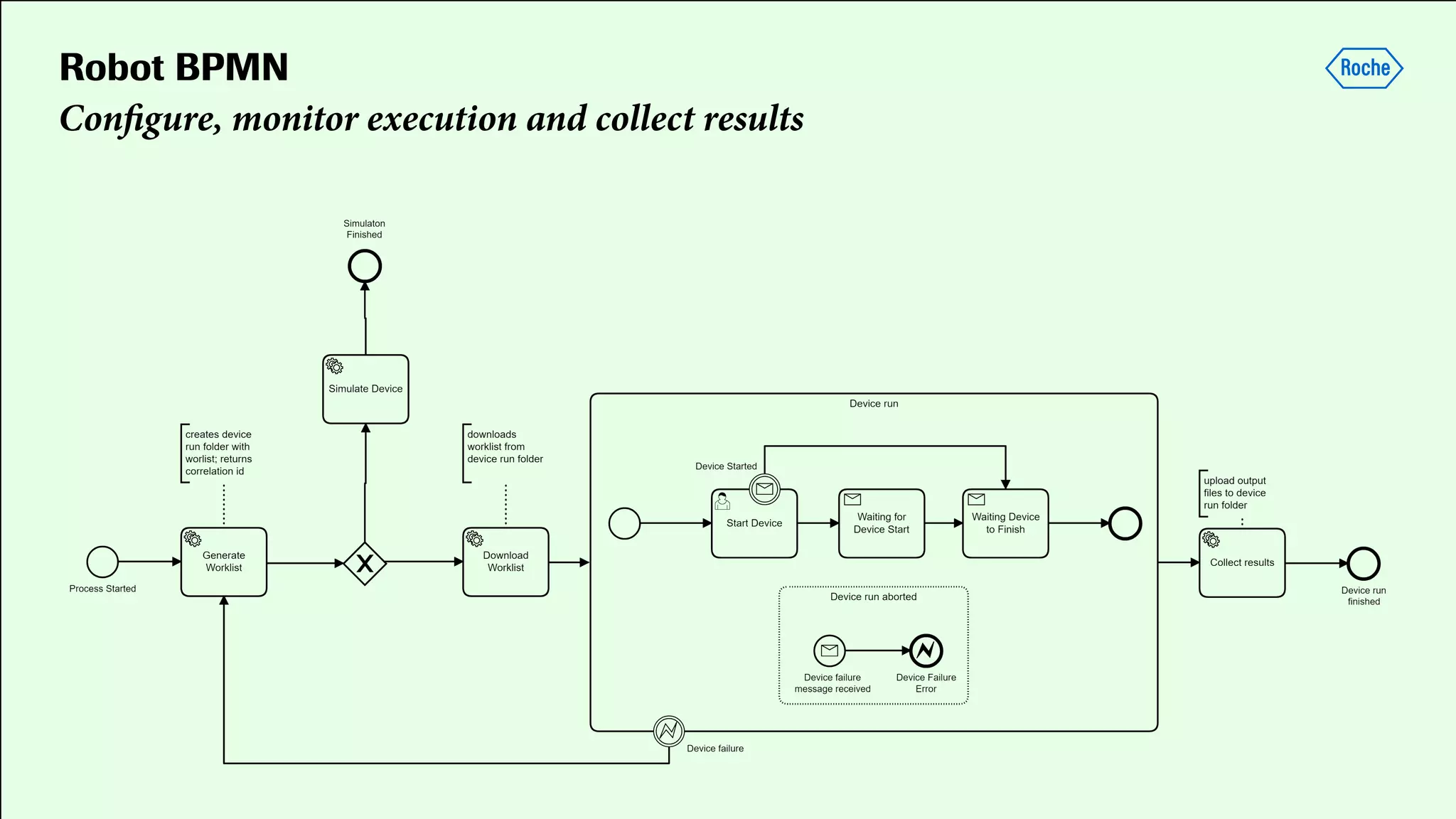
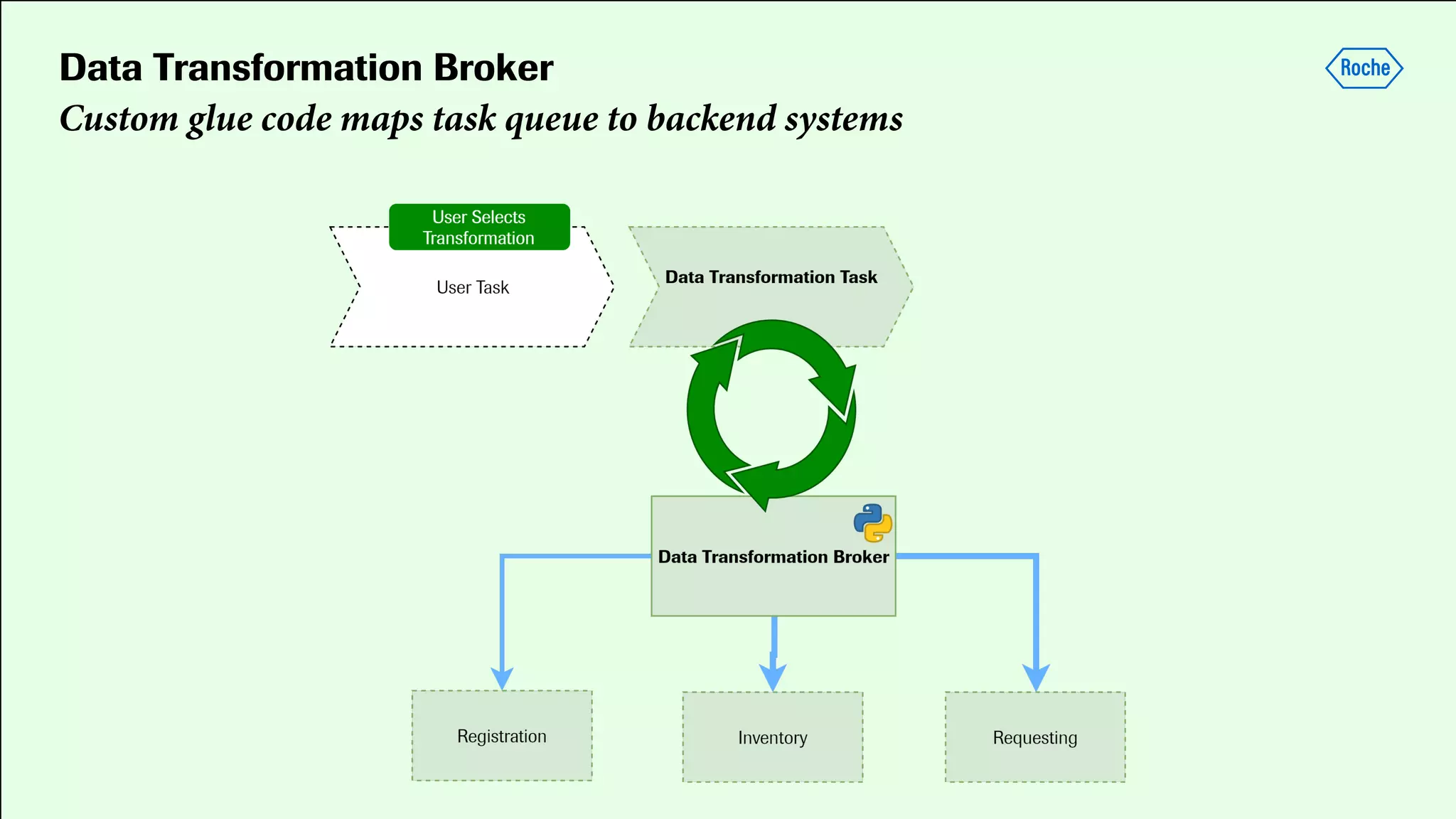
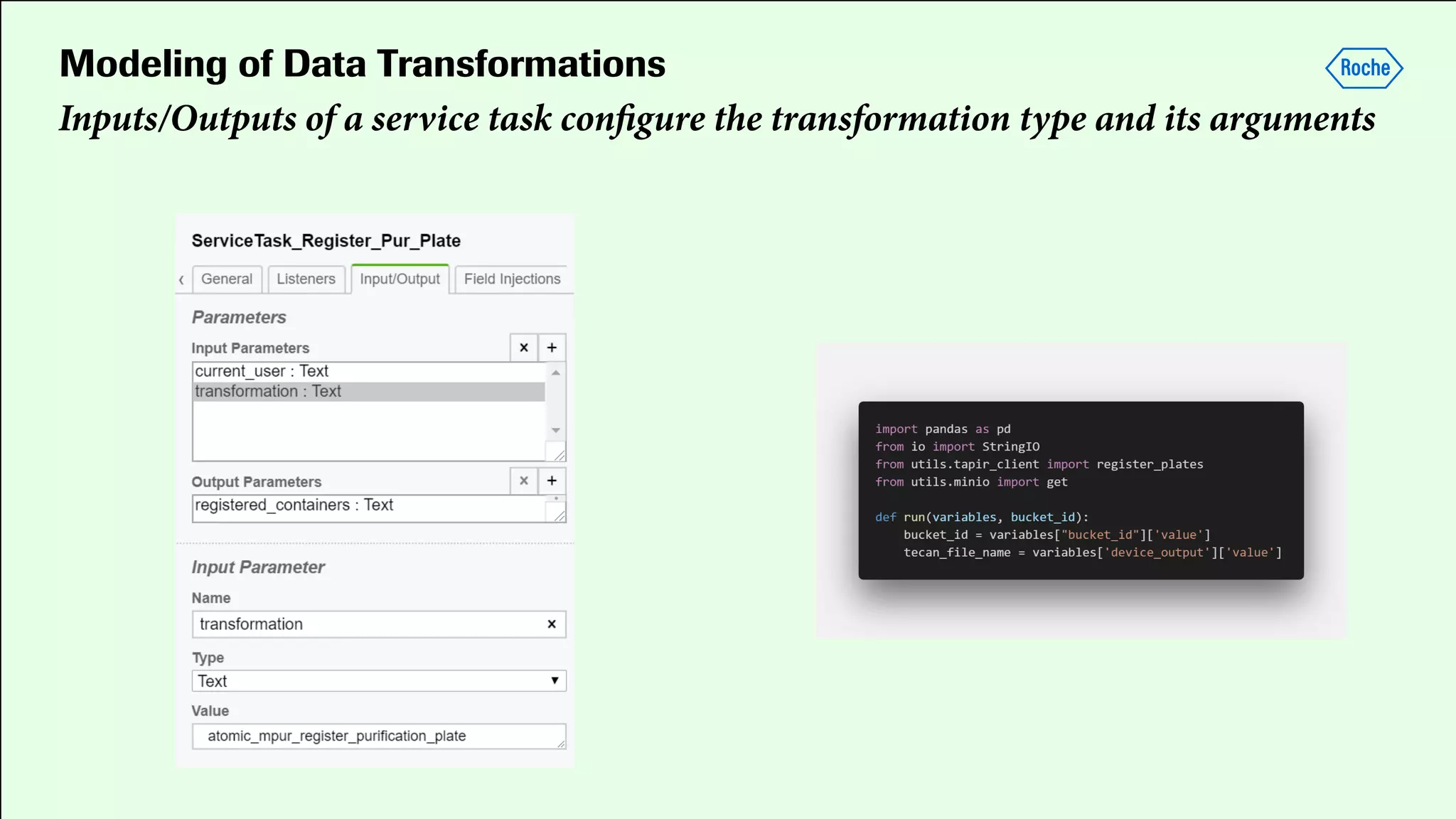
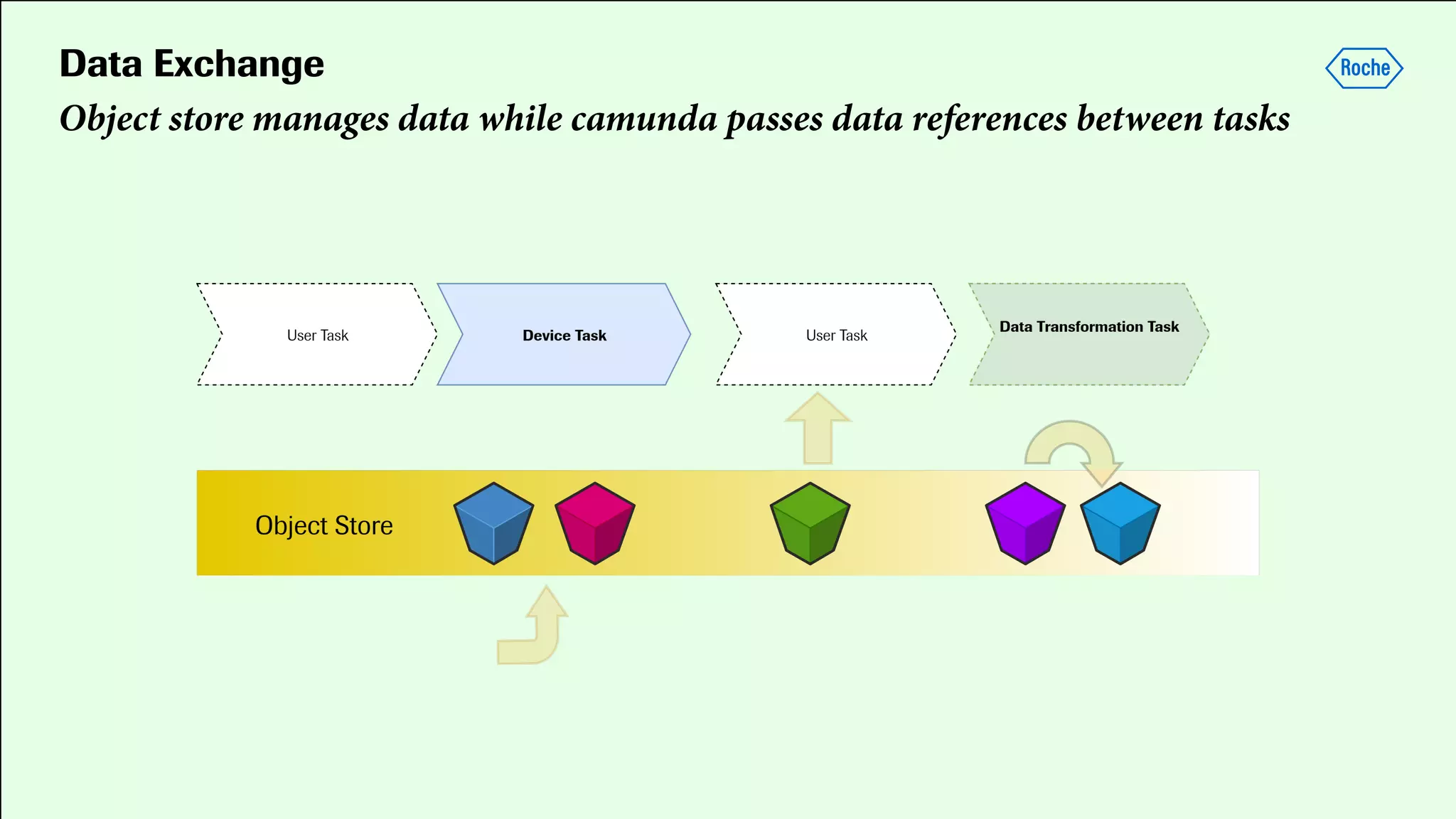
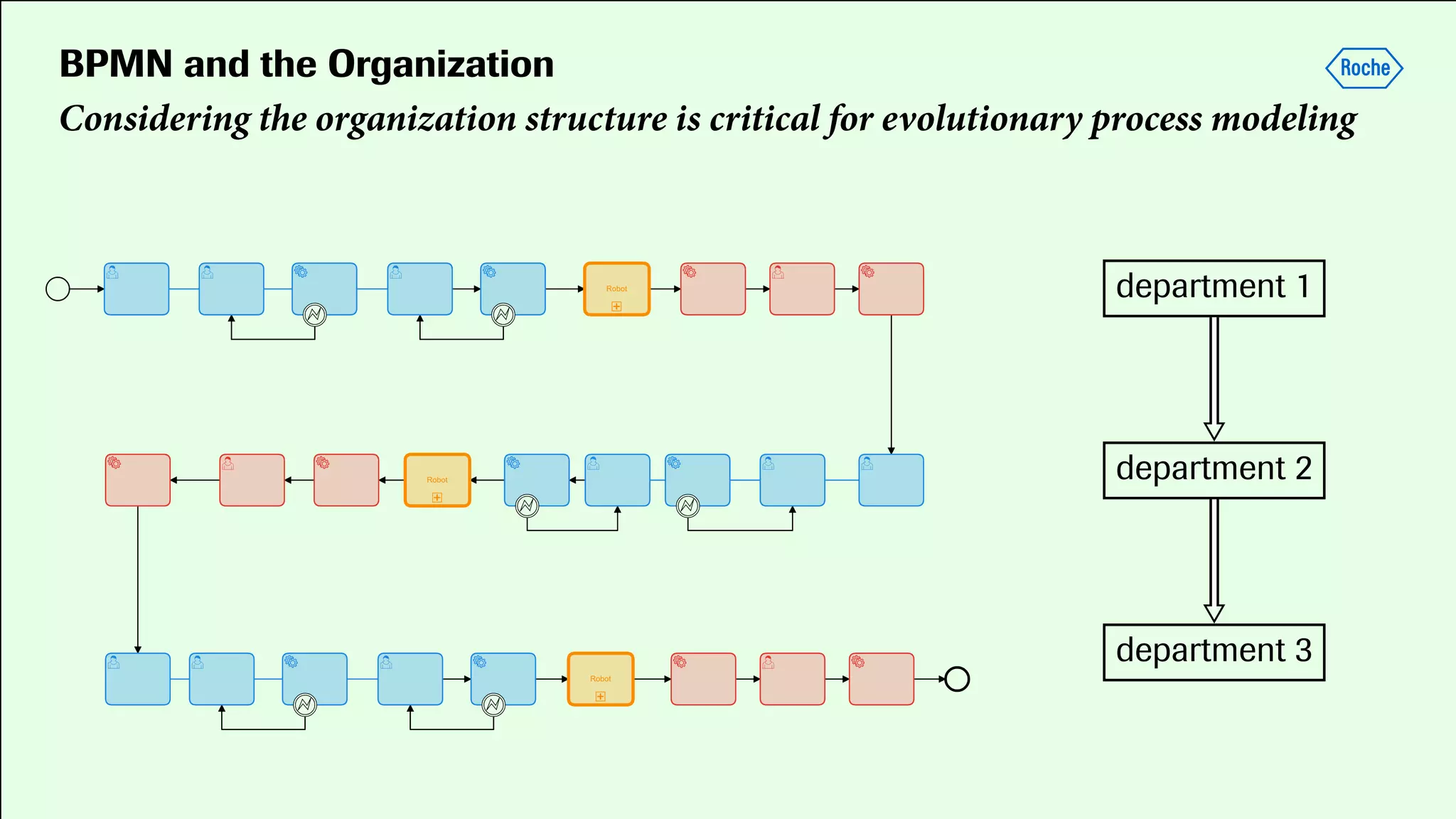
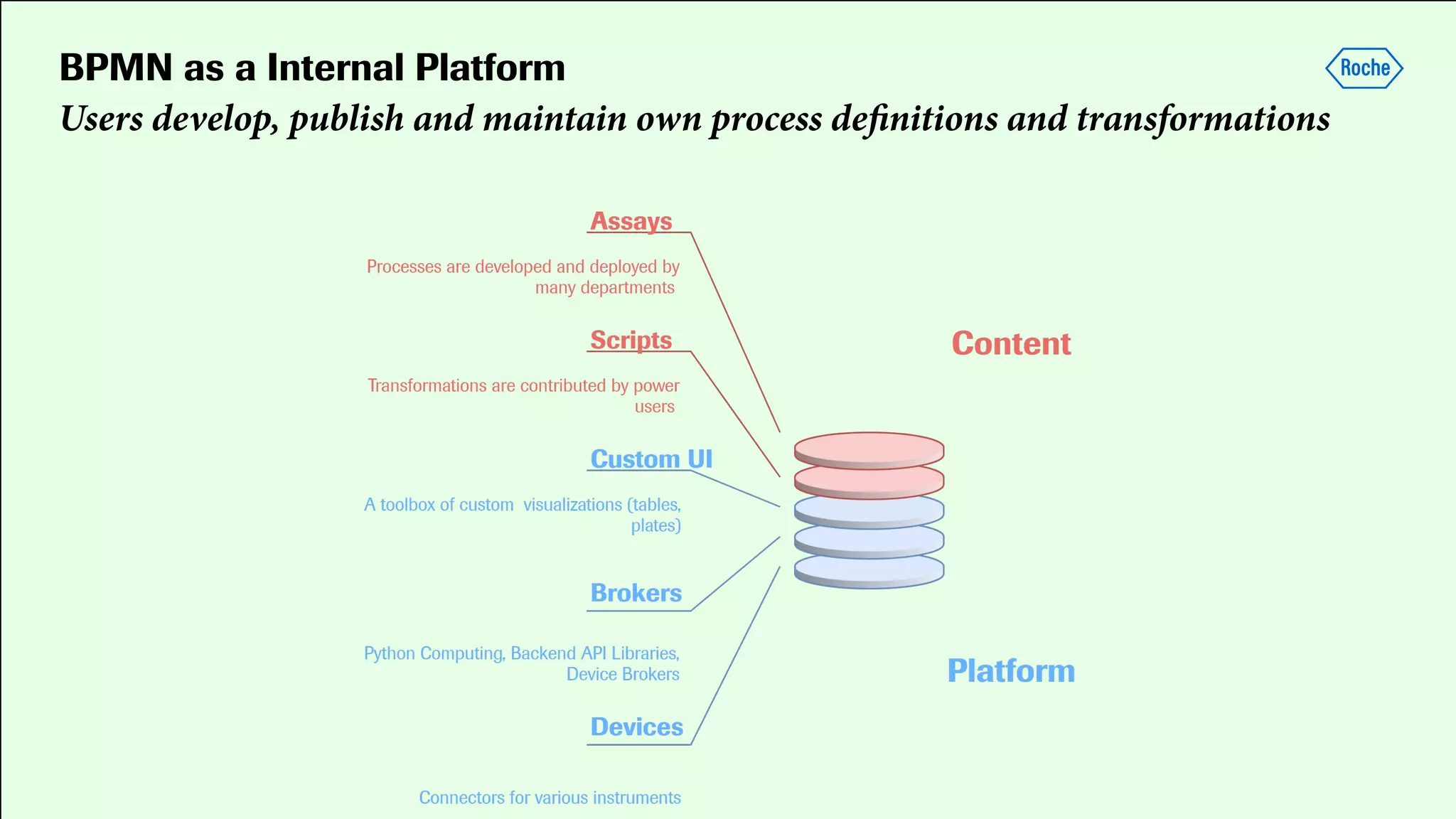
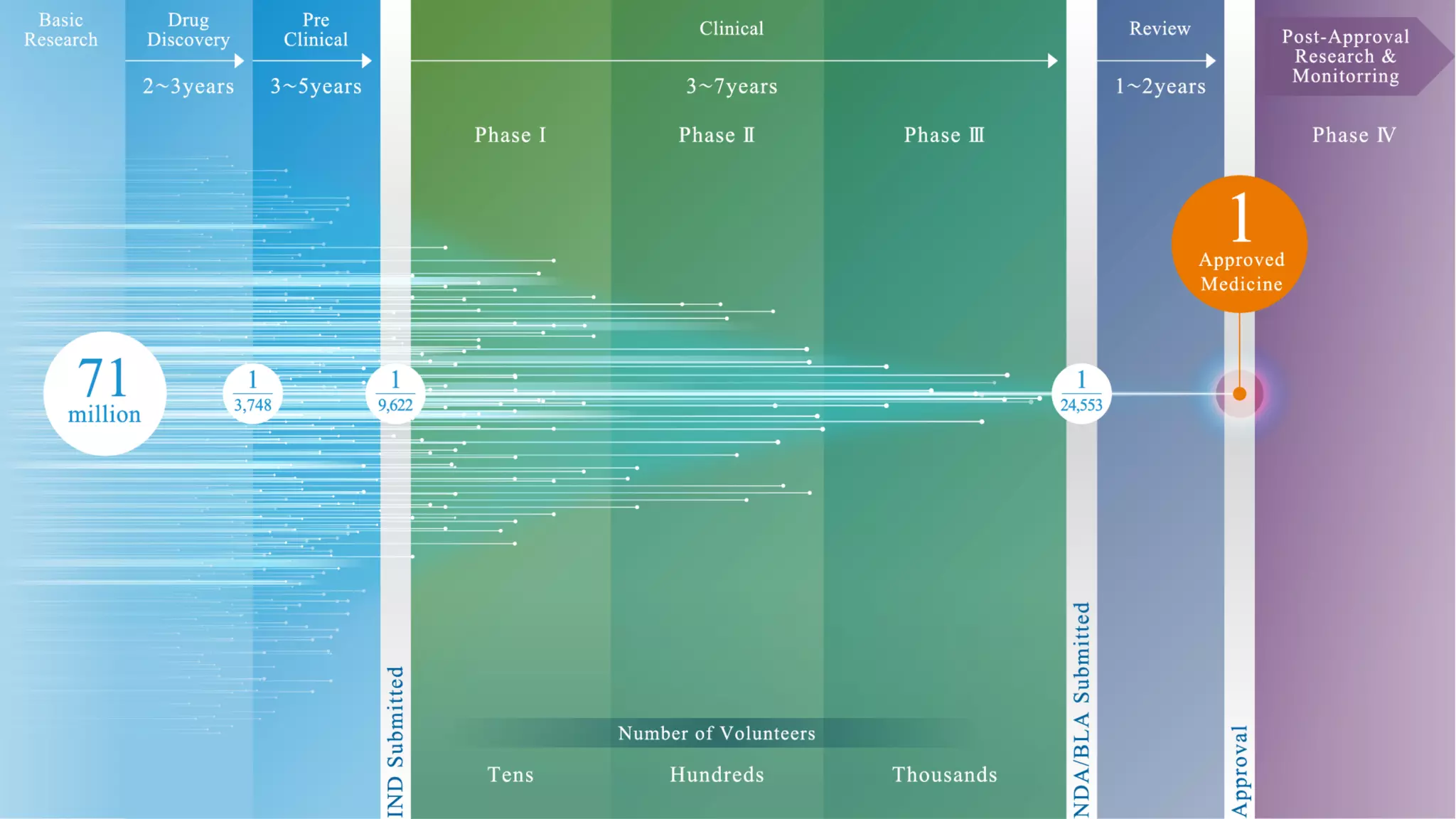
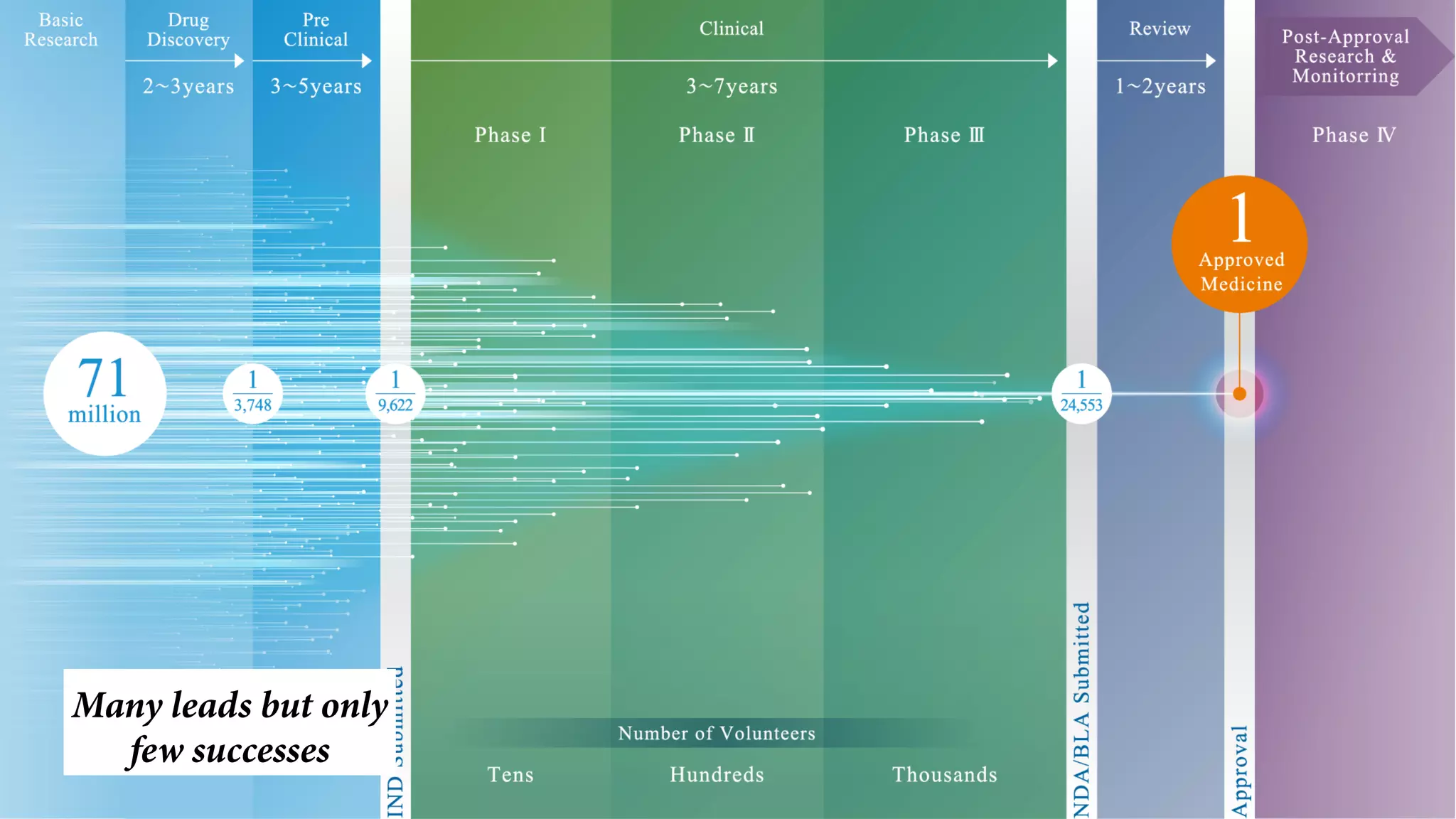
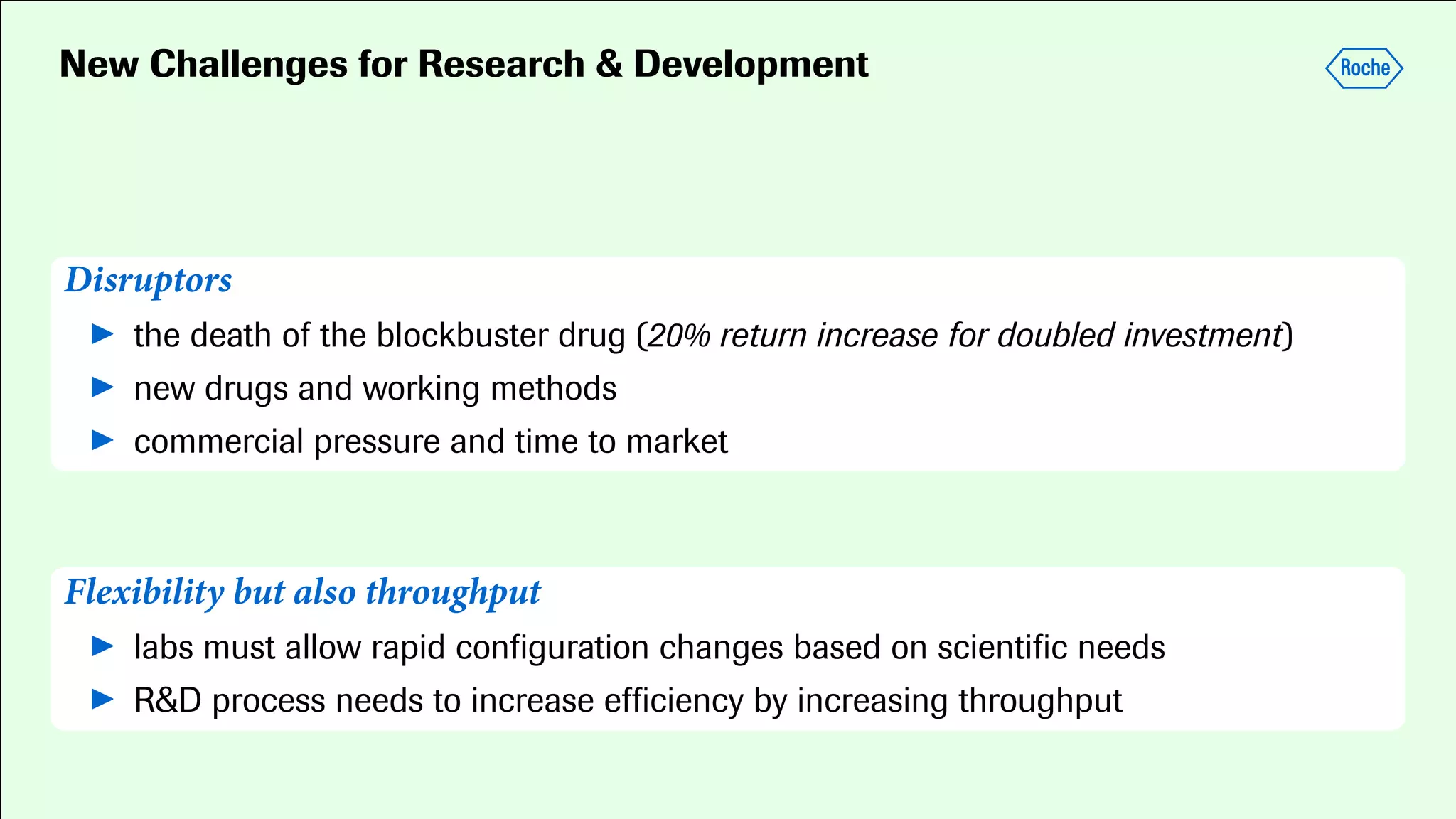
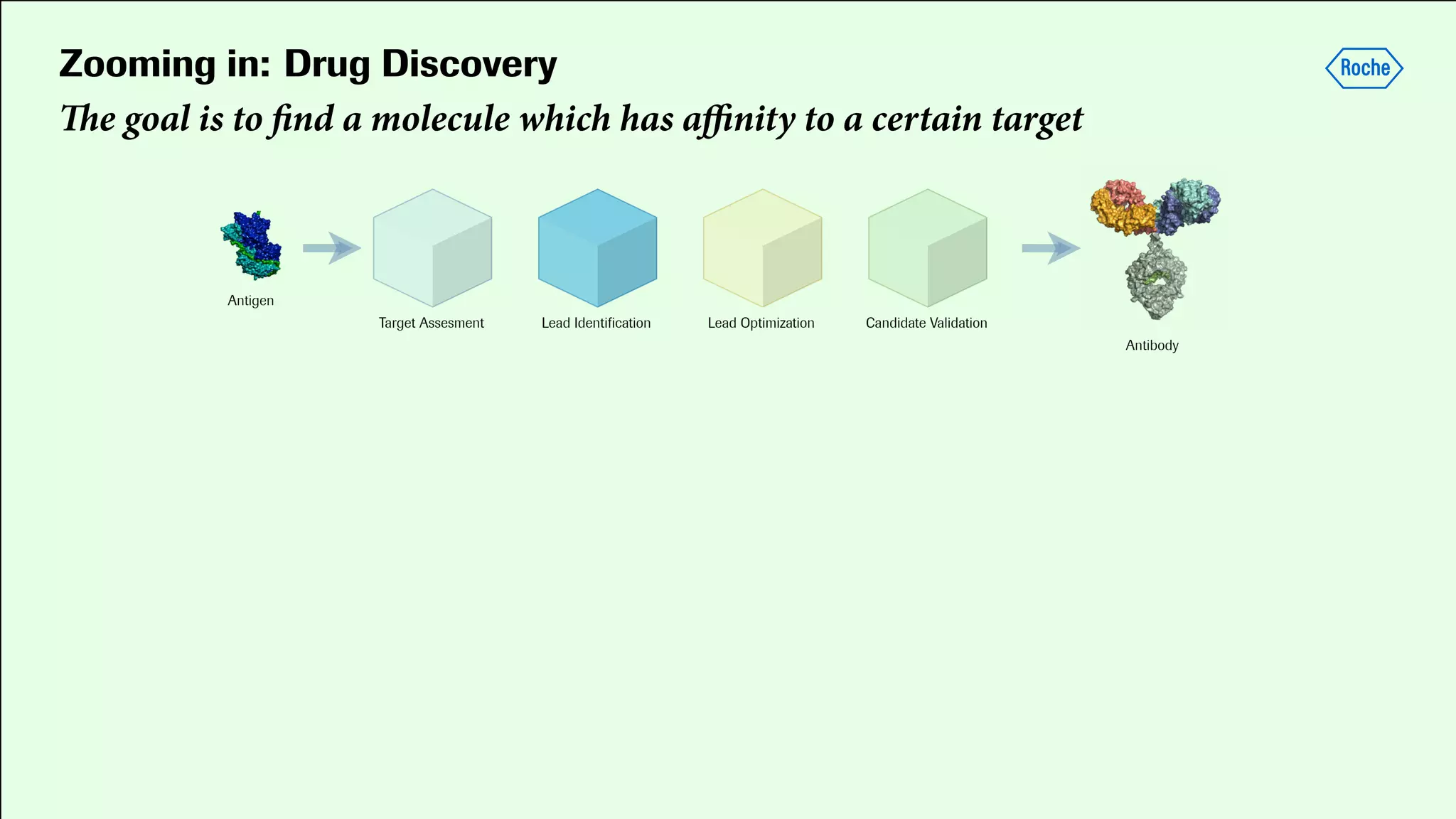
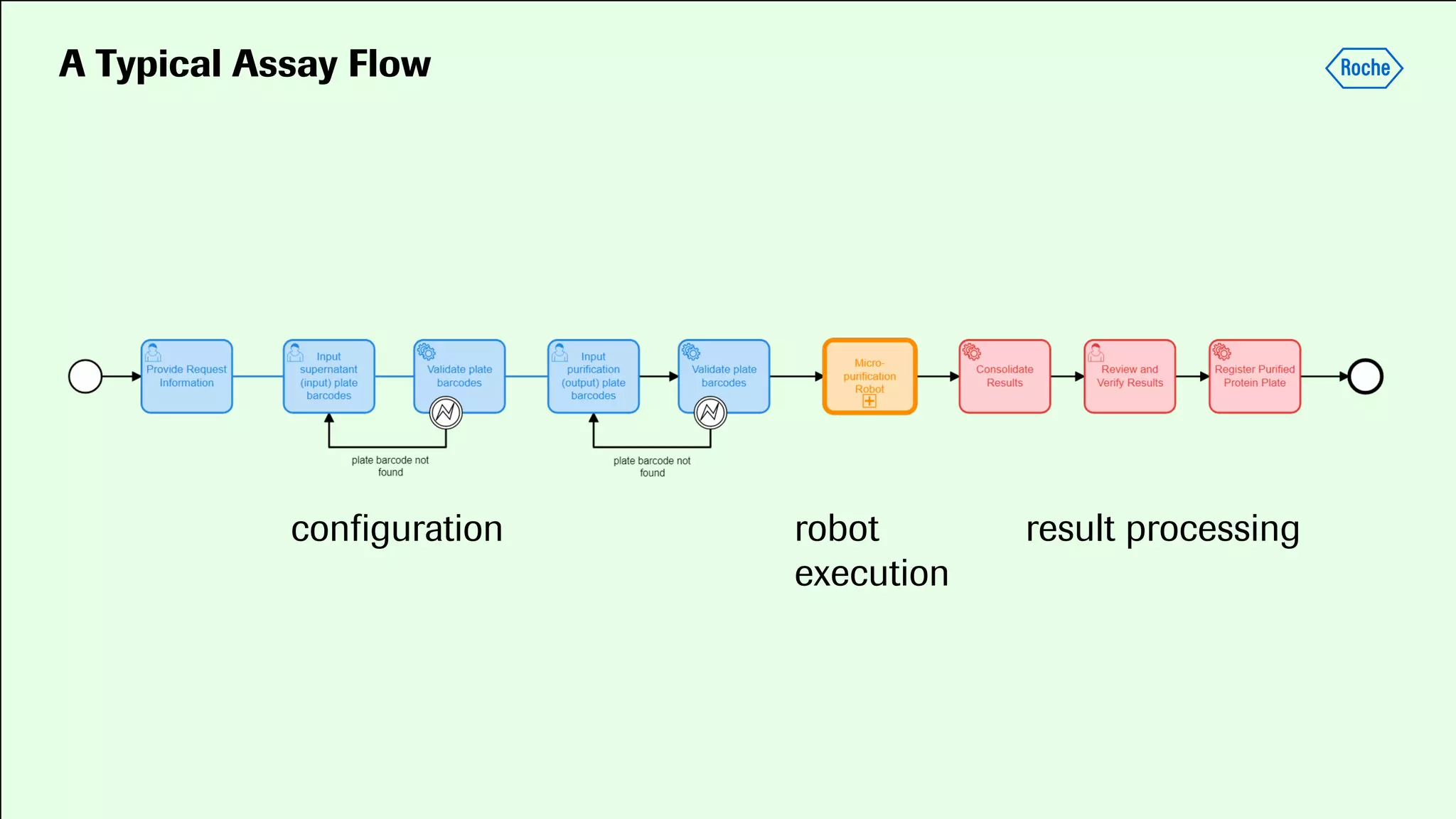
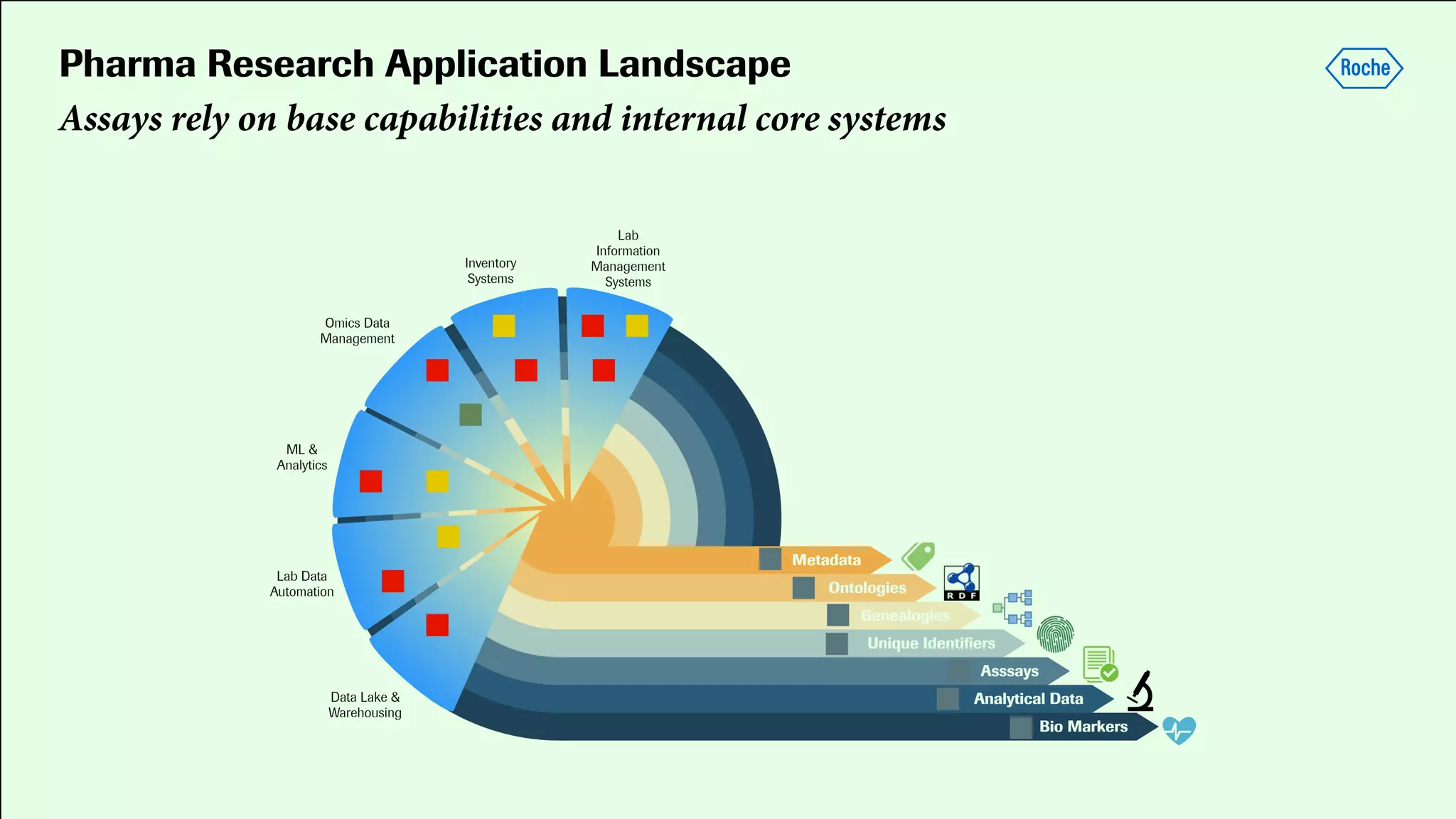
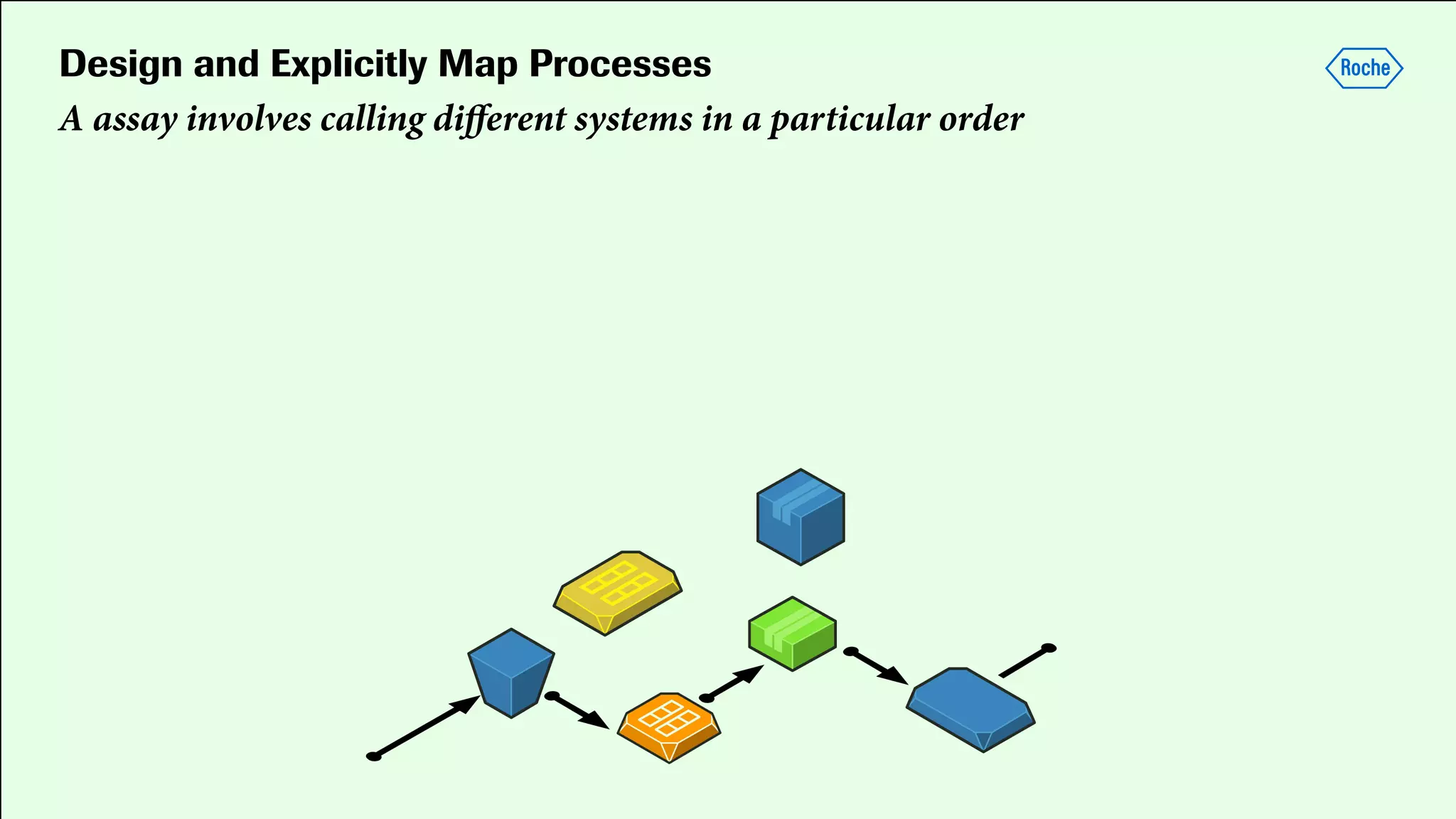
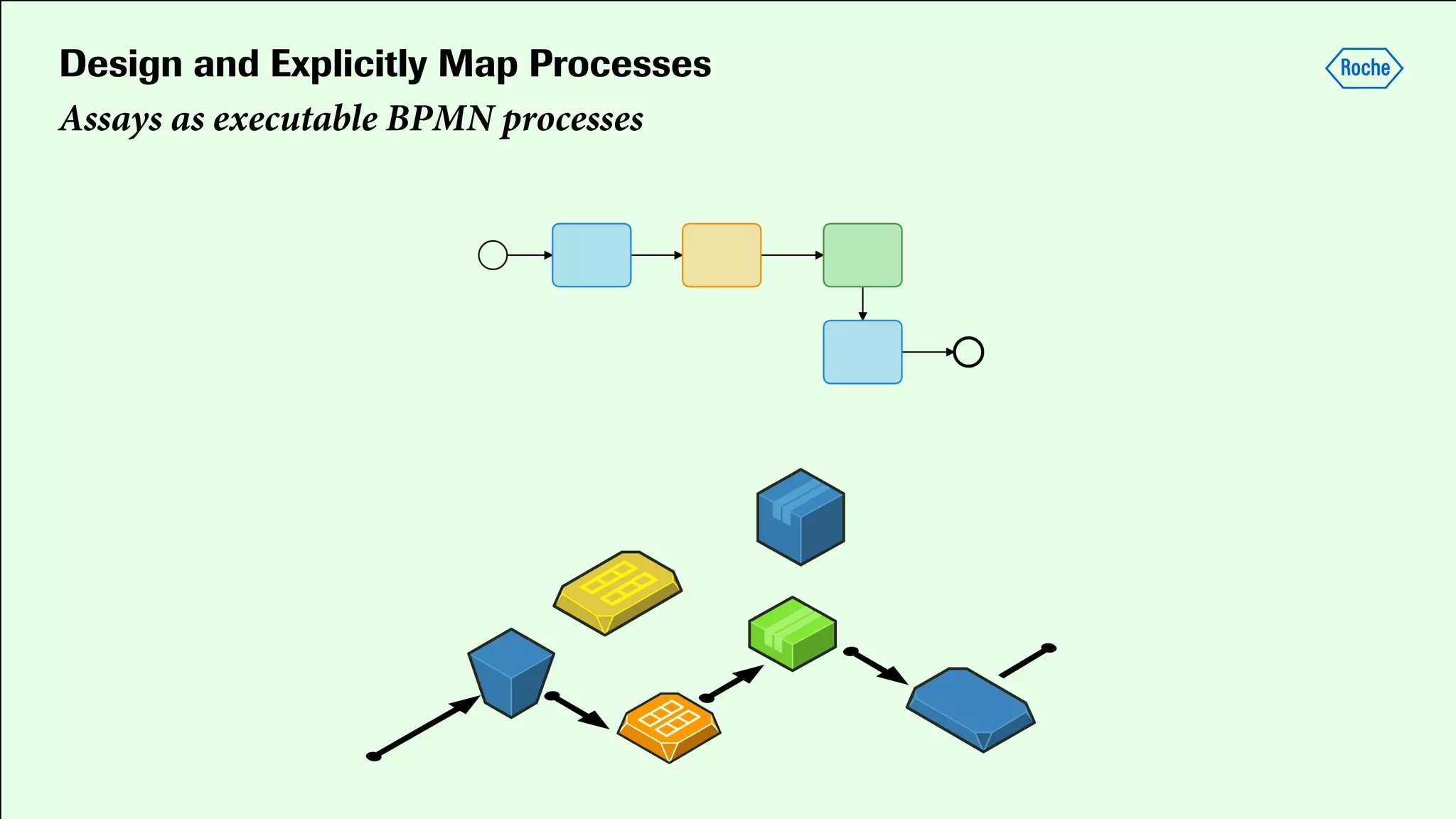
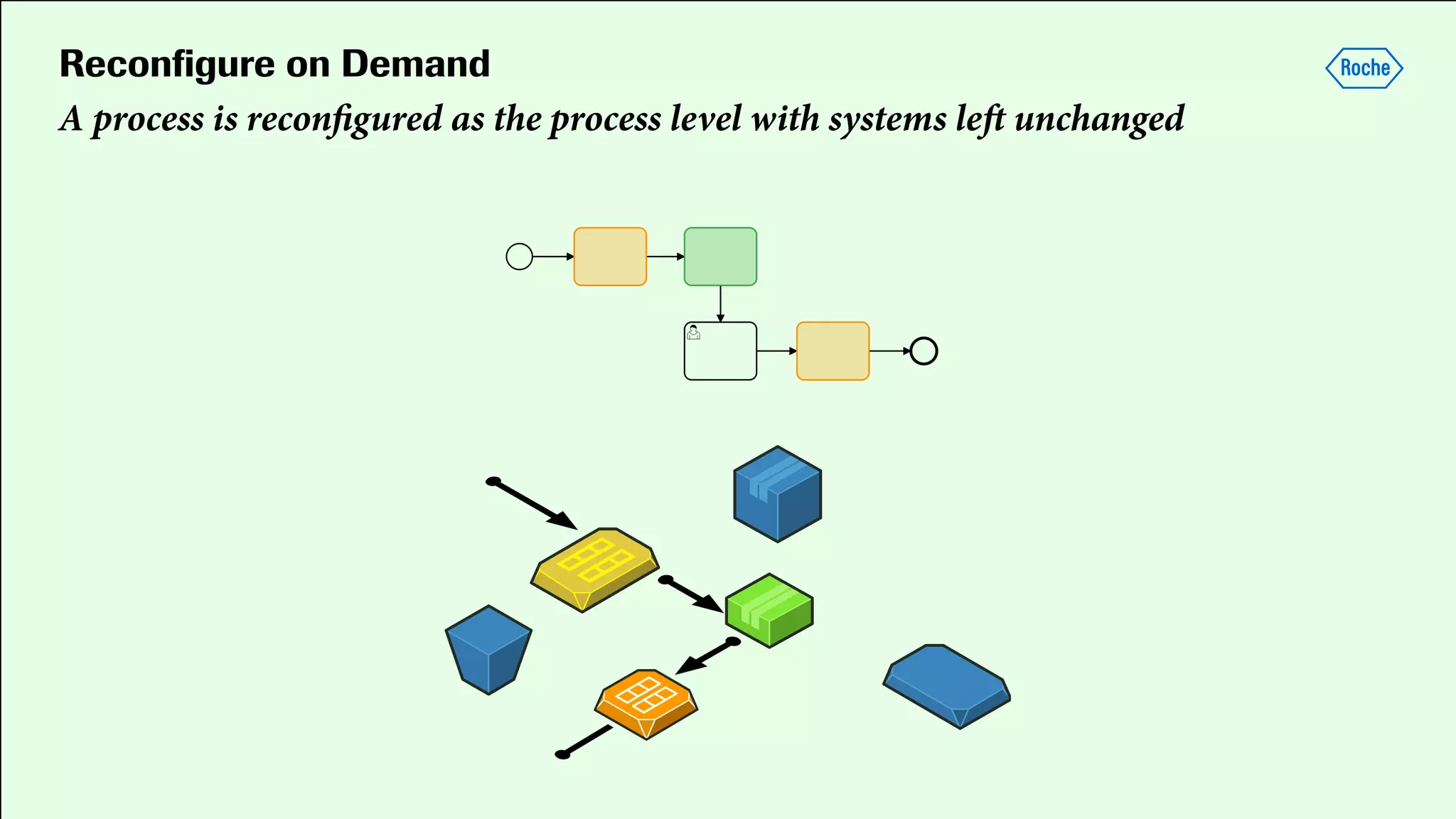
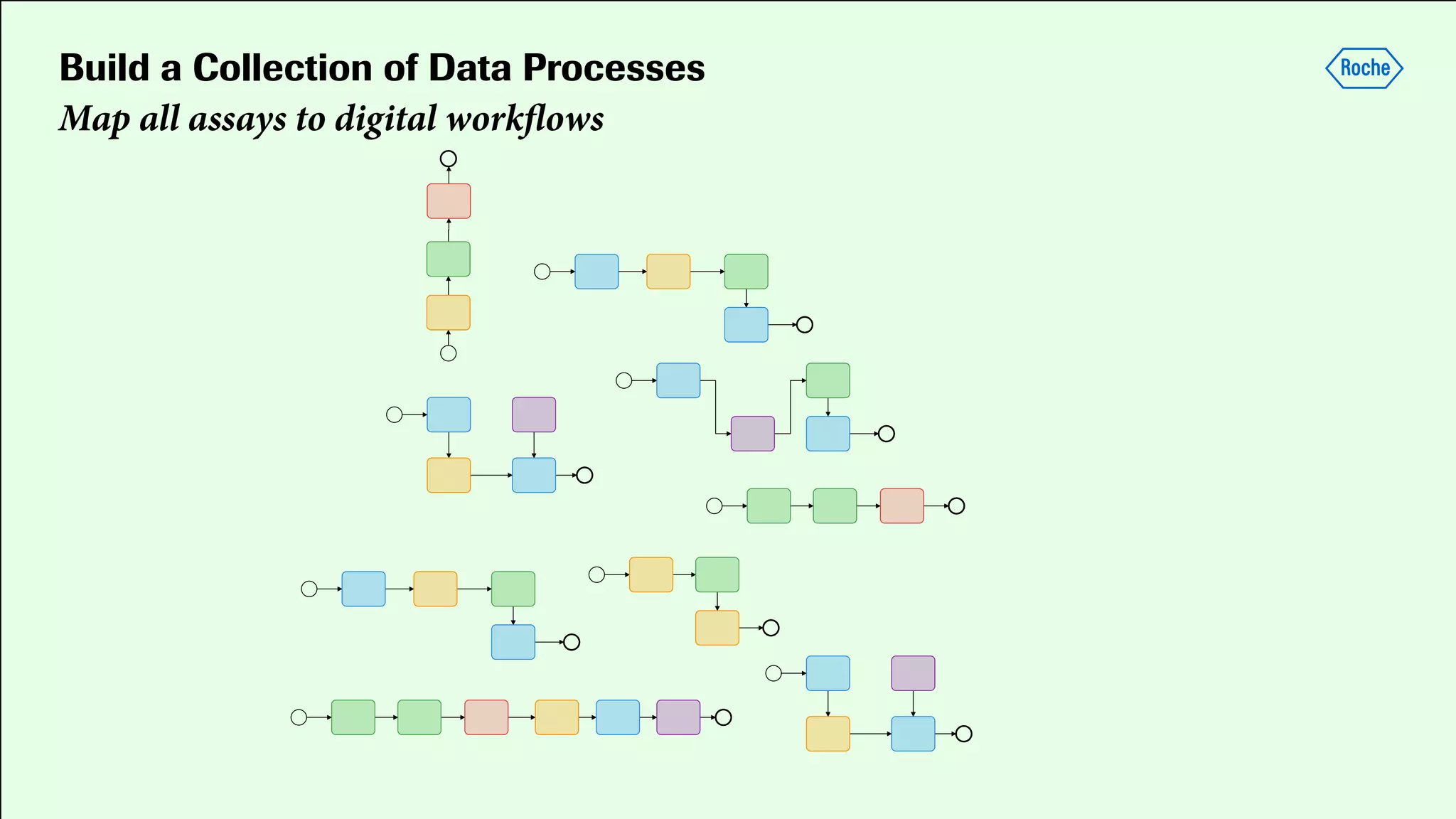
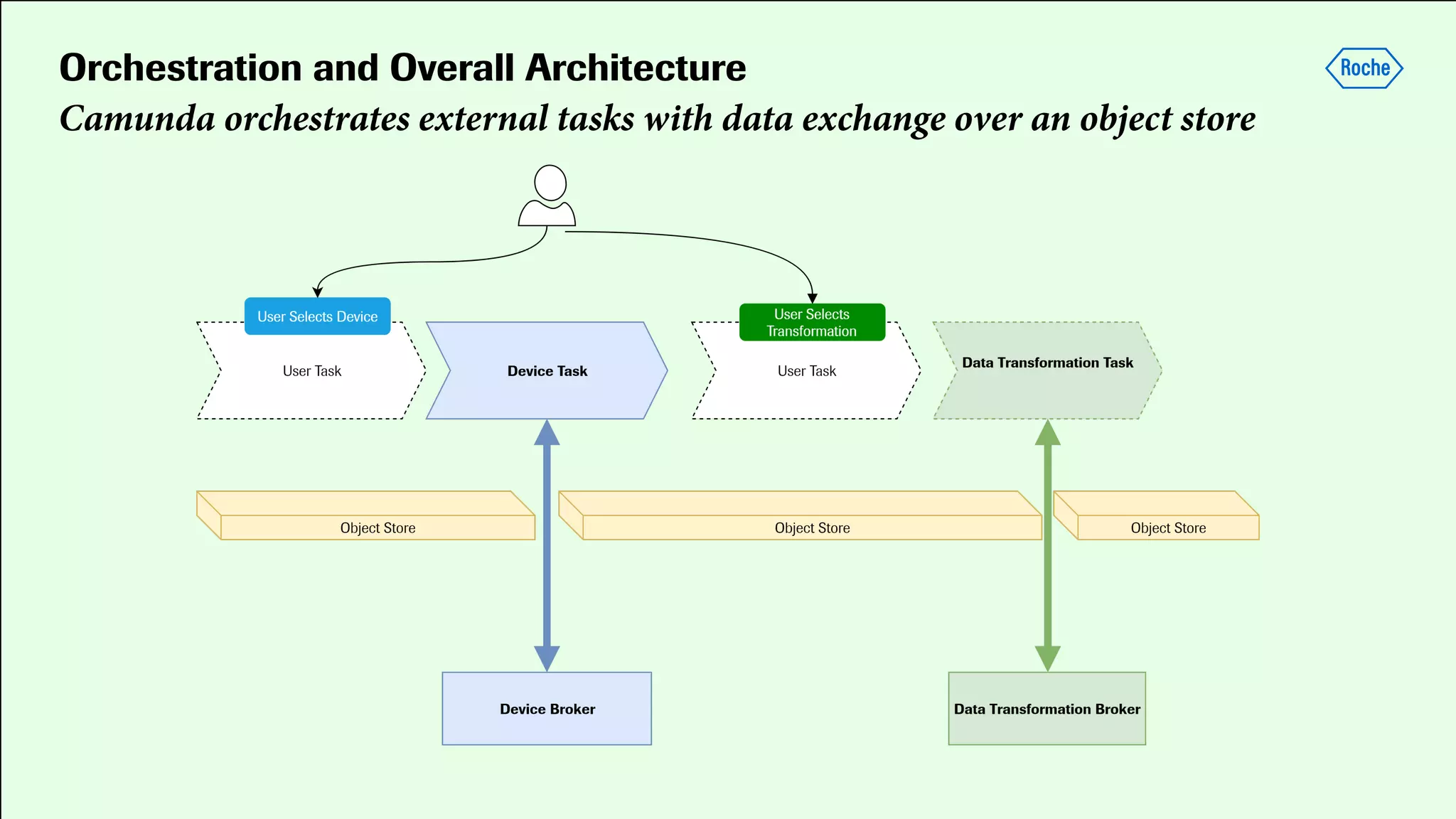
Pharmaceutical research faces challenges with a high volume of leads but few successful outcomes, necessitating increased efficiency and flexibility in drug development processes. The document discusses the implementation of automation, specifically through BPMN (Business Process Model and Notation) to create executable assays, enhancing the integration and orchestration of various devices and systems. By allowing users to model and manage processes internally, the industry can adapt and scale research efforts to meet evolving patient needs.






![Bruker Maxis [Mass Spetrometer]
Biomek i7 [liquid handler]
Thermo Scientific [Chromatograph]
Biomek i5 [liquid handler]](https://image.slidesharecdn.com/rochefinal-190918104756/75/Pharma-Research-Automation-by-Connecting-Researchers-with-Robots-and-Systems-by-Daniel-Butnaru-23-2048.jpg)