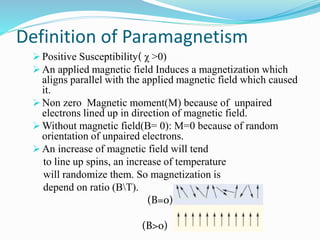
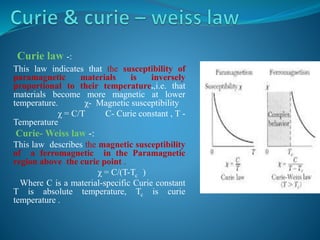
This document discusses different types of paramagnetism. It defines paramagnetism as having a positive magnetic susceptibility and being able to align with an applied magnetic field. Paramagnetism can originate from unpaired electrons or conduction electrons in metals. The document then covers Lengevin's classical theory of paramagnetism, Curie and Curie-Weiss laws, Pauli paramagnetism from conduction electrons, and the crossover between localized moment behavior and Pauli paramagnetism.



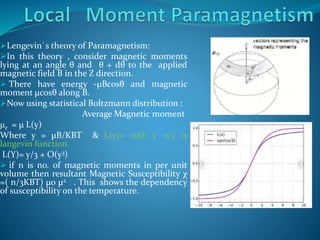
![In general, magnetization M =Ms Bj(y)
Where saturation magnetization Ms = ngj μBJ
Where Bj(y) = Brillouin function
By solving this ,susceptibility χ=(n/3kB T)μ◦ μeff
2
μeff is effective magnetic moment = gj μB
[J(J+1)]1/2
where Lande gj value = (3/2) + S(S+1) –L(L+1)
2J(J+1)
Using hund`s rules application, For 4f
elements, effective magnetic moment value closed
to experimental value .
3d elements experimental effective magnetic
moment are not agree with this theoretical value
because of Orbital quenching it means orbital
angular momentum is quenched (L=0).](https://image.slidesharecdn.com/paramagnetismproperties-230807105949-bfccb05c/85/Paramagnetism-Properties-pptx-6-320.jpg)

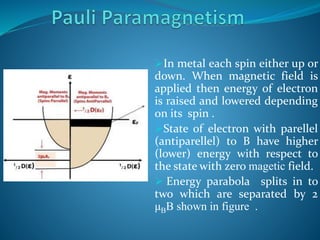

![ The effect of the Fermi Dirac statistics and the croos over Pauli
paramagnetism between localized moment behavior can be illustrated by
rederiving .
n+ =1/2ʃg(E+μBB)f(E)dE
n- = 1/2ʃg(E-μBB)f(E)dE
Now Magnetization M = μB (n+ - n- )
Now solving this using Taylor expansion,
M= μB
2 Bʃ(-df/dE)g(E)dE
In the degenerate limit at T=0, M = μB
2 Bg(EF ) & χp = μ◦g(EF)μB
2
In the nondegenrate limit f(E) =exp[-(E- μ)/k B T] so by solving this :
M = n μB
2 B/K B T & χp = nμ◦μB
2 /K B T
This susceptibility equalnt to n localized moments (of magnitude μB )
per unit volume .](https://image.slidesharecdn.com/paramagnetismproperties-230807105949-bfccb05c/85/Paramagnetism-Properties-pptx-11-320.jpg)
