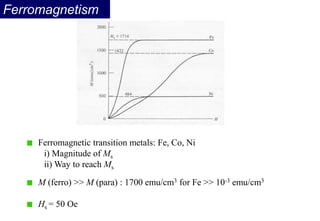
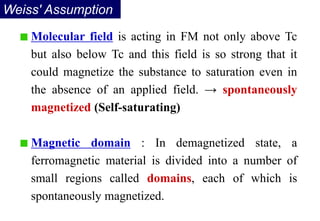
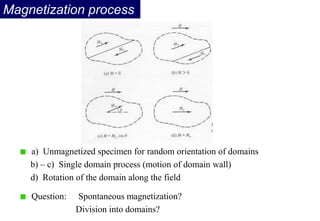
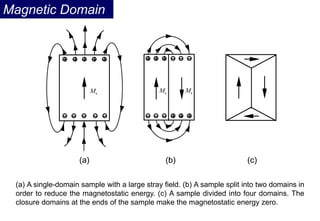
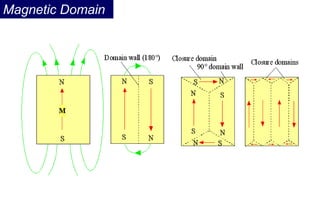
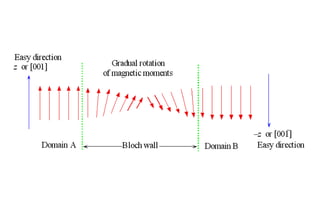
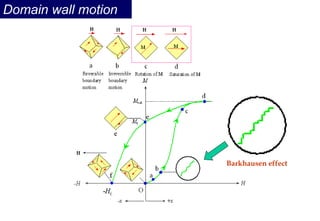
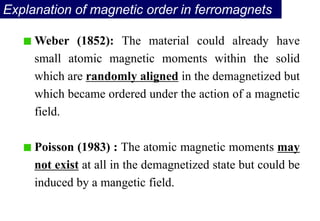
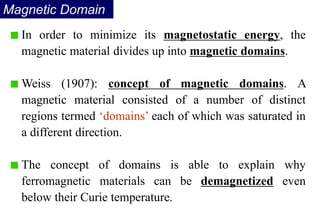
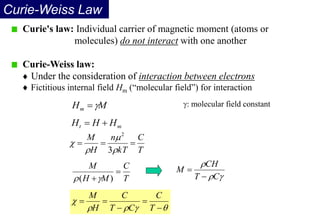
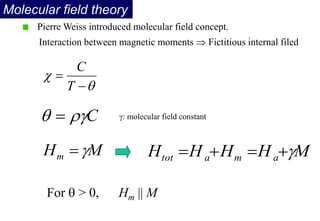
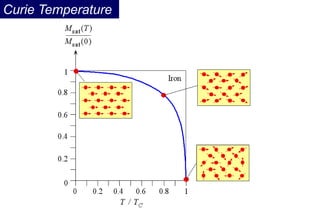
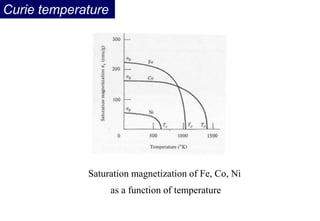
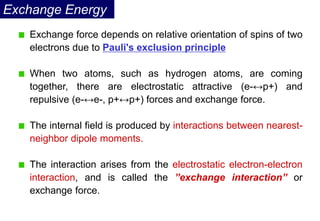
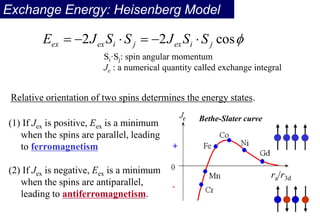
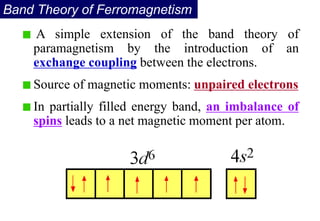
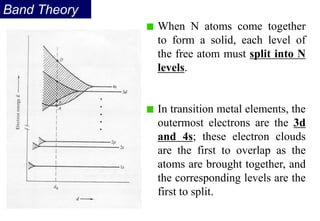
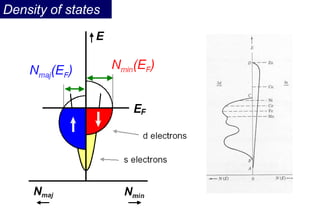
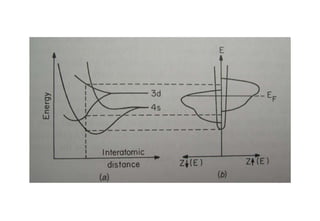
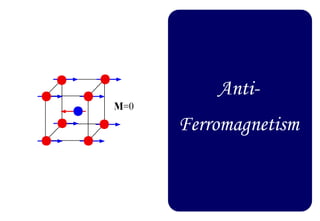
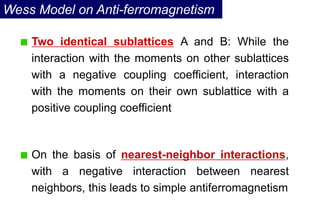
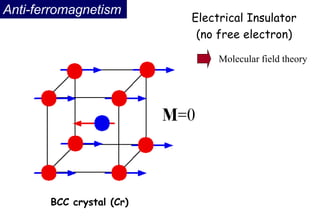
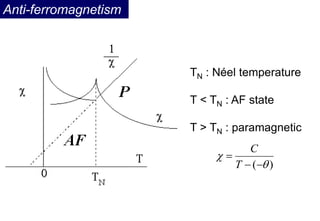
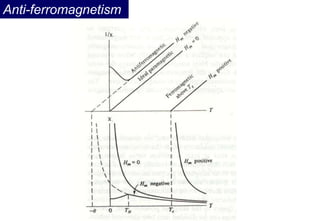
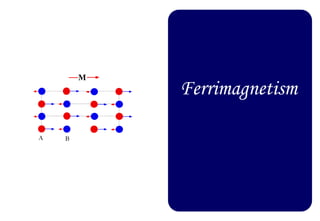
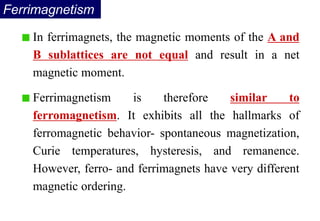
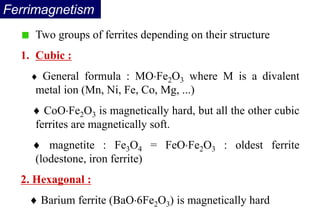
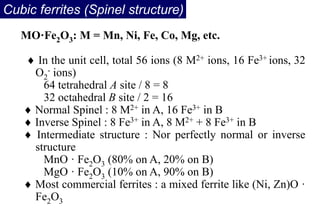
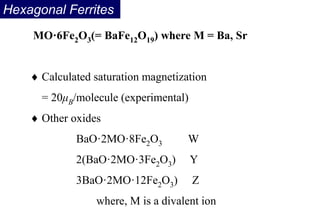
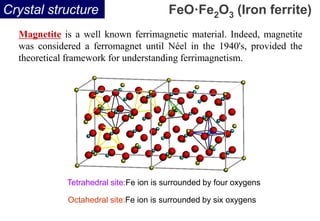
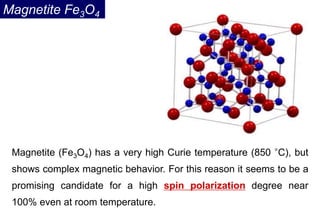
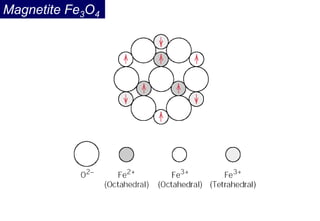
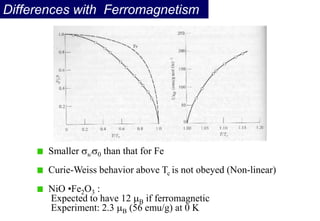
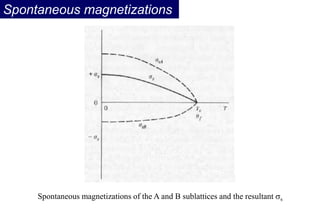
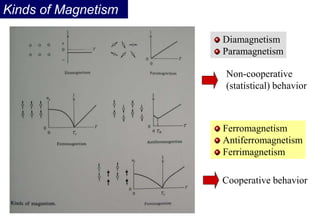
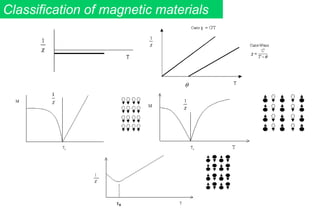
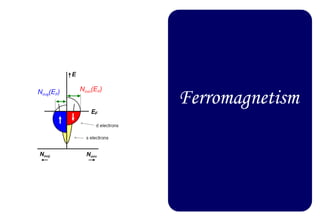
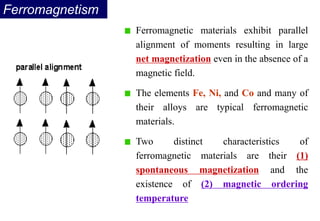
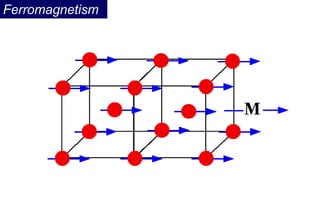
Ferromagnetism arises from the parallel alignment of atomic magnetic moments due to strong exchange interactions between electrons. Ferromagnetic materials can exhibit spontaneous magnetization and magnetic ordering temperatures. The exchange force is a quantum mechanical phenomenon that favors parallel spin alignment of electrons and results in large internal molecular fields that align atomic moments. Ferrimagnetism is a similar phenomenon where sublattices have unequal and opposing magnetic moments, resulting in a net magnetic moment. Common ferrimagnetic materials include ferrites with spinel and hexagonal structures.

![Hund’s rule
Fe: [Ar]3d64s2
Origin of Ferromagnetism](https://image.slidesharecdn.com/2-221028093707-c8b99aab/85/2-Magnetism-1-ppt-5-320.jpg)