This term paper discusses paper-based disposable analytical devices, emphasizing their portability, ease of use, and applications in biomedical and environmental fields. It highlights various fabrication methods and detection techniques, such as colorimetric and electrochemical detection, which enhance real-time onsite analyses. The conclusion calls for further development to bring these low-cost tools into practical use, particularly in developing countries.


![3
Introduction
In our daily life paper is used for writing, drawing, printing and packaging. Paper has unique
physical properties that allow to be used other applications unlike the well-known traditional
means. It is a light, thin and flexible material. Paper mainly consists of cellulose fiber which is a
good platform for certain applications. The hydrophilic fiber in paper allows liquid to pass
through without using pumps or external force to bush the liquid. In addition the cellulose fiber
can be manipulated with the desired property .for instance, the hydrophobicity can be changed
and the rate of movement of fluid through the fiber.[1]
Nowadays researchers did a lot of work on paper especially for analytical devises, sensors and
clinical chemistry. This is because paper is flexible, available and low cost. Analytical devises
based on paper are inexpensive, disposable, portable and simple to use. After the paper
chromatography has been invented in 20th
century paper based diagnostic devises began to
emerge.
Paper passed analytical devises for the quantification of glucose and urine was the first devises to
be discovered. Next came the well-known pregnancy kit. The main structure of this devises
consists of a strip of paper with a place to introduce the sample (sample pad), reagent pad and
test line (Fig 1). The sample containing the antigen runs through the paper through capillary
action, it binds with the antibody signal indicator to form antigen/ signal this gives a positive
result. The signal indicator is color and the detection result is mainly yes or no.[2]
Fig 1 A quasi “all-inkjet-printed” μPAD FIG 1. μPAD fabricated by photolithography](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-3-320.jpg)
![4
Microfluidic paper based analytical devises have benefits better than the traditional glass and
polymer based microfluidic chips. Paper is made from cellulose in aqueous dilute form. The
suspension of fiber is sieved, and by pressing and drying to produce a sheet of randomly formed
cellulose fiber. The fibrous and porous structure of paper provides the following properties. 1)
No need of external force to bush fluids through the paper simply by capillary actions fluids
mover through the paper. 2) Good absorptivity, this allows to hold the reagent in place. 3) Shows
good permeability, no problem with air bubbles. 4) The random network structure of the celluse
fiber enables the filtration of the sample. 5) it has high surface area to volume ratio , this creates
more volume to immoblise reagents. [3]
Recently paper has become excellent platform for lab-on-a-chip analytical devises. In this
devises thousands of tests and complicated laboratory tests could be carried out. In addition, they
enable portable onsite real-time experiments which are important in many areas of applications
such as medical, food and environmental sections. In these areas, simple, compatible and
practical analytical devises are highly recommended. Because nowadays healthcare services is
getting more expensive and also many developing countries are lacking sophisticated clinical
tests therefore, inexpensive , fast and reliable POC paper based devices is needed. [4]
DISCUSSION AND RESULTS
1. Methods of fabrication of paper based
What determines the choice of fabrication technique is the cost and efficiency. There are a
number of physical and chemical techniques of fabrication that has been reported in the
literature. These includes, inkjet printing, wax printing, photolithography, plotting and laser
treatment. Almost all publications involve making only certain channels for the liquid to contain
in the paper and this is what is known as microfluidic paper-based. [5]
1.1 Wax patterning.
Wax is inexpensive and widely available material. It has long been used in μPAD, because it can
be applied and printed by different ways. Whitesides and coworkers at Harvad university
proposed a new method of wax printing during the early developments of μPAD , wax printer
will first make the pattern of wax on a filter paper and then the paper is heated on an oven to
allow the wax melt down into the paper through the porous structure of the paper. In this way a
micro Chanel is created [2]](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-4-320.jpg)
![5
1.2 Inkjet printing
The process of ink jet printing starts soaking a piece of paper in polystyrene dissolved in toluene. Then
the paper is removed and allowed the solvent to dry, the paper becomes hydrophobic. After that, and
inkjet printer makes pattern of the test lines with toluene. Repeated moves remove the polystyrene
deposited areas and create hydrophilic micro channels. [2]
Fig 2 wax patterning.
Fig 3 Inkjet printing.](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-5-320.jpg)
![6
1.3 Photolithography
In this technique the paper is patterned with photoresist (SU-8 210) . Then it is exposed to UV
light (405nm). The light passes through the photo mask. After the exposure it is dried. The
photoresist is washed with propan-2-ol. Next reagents are applied into the paper. The photo resist
is expensive, but later on researchers used much cheaper type of photoresist.[4]
.
Fabrication methods of 3D μPAD
In order to meet the need for faster and efficient analysis or carry out multistep chemical reaction
and multiprocessing steps on one chip, researchers have come up with 3-DμPAD. 3D-μPAD can
be developed by staking alternating layers of 2-D μPAD. Three dimensional μPAD are more
beneficial than 2D-m PADs for a number of reasons. First, faster follow speed; this is because
the distance traveled by the liquid along the z axis is shorter than the distance the liquid travels
along the x y plane. Secondly, each layer of the can be used to carry out specific function for
instance one layer will do filtration while other layers can do the identification. Finally the
overall fabrication is simple because it is originally come from the 2-D μPAD. [6]
Fig 4 photolithography](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-6-320.jpg)
![7
2. Detection Methods
2.1 Detection by colorimetric.
This type of detection is one of the oldest and most commonly used detection methods in μPAD
is colorimetric detection especially in semi-quantitate results or yes or no answers. It depends on
the visual detection of the eye. For instant, in urine analysis the detection of glucose and protein
is colorimetric . Once the sample touches the detection zone, color change appears duet to the
enzymatic reaction where by the positive result is seen when the color changes from colorless to
brown. While the protein color changes from yellow to blue for the positive result.
A disadvantage for this method is that the eye is not very accurate to differentiate some colors
and ambient light is required always for good observation. This problem can be solved by using
camera phones or scanner connected to computer. The image pixels represent the analyte
concentrations. Recently the pregnancy test kit devise also uses this type of detecting color
change. This devise is actually inexpensive , fast, stable and reproducible [4][1]
2.2 Electrochemical Detection
In many research papers, electrochemical detection has become one of the most investigated
detection method in μPAD. The glucose detection using screen printed electrode was one of the
most successful results of this technique. To develop such devises an additional step in the
fabrication method is required which is the deposition of electrodes.
Fig 5 .three dimensional 3d
fabrication](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-7-320.jpg)
![8
Unlike the colorimetric, electrochemical detection gives faster, more selective and sensitive
detection. It can give to lower detection limits compared to colorimetric. Moreover, it does not
respond to lightening conditions and contaminants. However, further research is needed for the
miniaturization of this devises to be suitable in many other applications. [2]
2.3 Fluorescence sensing
This technique can be used analytes that can absorb light and give fluorescence properties. So far
many μPAD with fluorescence detection has been proposed and achieved lower detection limits.
Nevertheless, this type of techniques suffers from severe interference from the substrates that
may contain some additives that are fluorescing active. Generally speaking μPAD has been used
in many applications such as detecting proteins, cancer cells, drugs and others. However, these
techniques should be low cost and miniaturized. [4]
3. Onsite analysis and Applications
A major advantage of μPAD is that they are portable. In this feature they enable end users to do
analysis onsite and real time analysis. No need of taking samples to the labs but instead samples
are analyzed on the spot. Moreover, the risk of sample loss or contamination is minimized and
also does not require preserving sample for long time. On-site determination of analtytes using
μPAD makes response time faster and with lower cost. It is worth mentioning that the detection
method should also be portable. There are several methods which can make this possible. For
instance, smartphones and portable scanners. [7]
3.1 Environmental Applications.
Many μPAD based on colorimetric method of detection have been reported to detect and
quantify environmental pollutants. This is because μPAD are portable and easy to use. These
environmental contaminants might be organic, inorganic ( metals and nonmetals) or biological
(bacteria). Regulatory agencies such as US EPA and OSHA have already established guidelines
for the exposure to toxic chemicals. [3]
Heavy metals such as Cr6+, Hg2+, Ag+ Pb2+ Cu2+, Fe3+, and Ni2+ can be measured using
colorimetric paper based sensors. These guys are really alternative to AAS and LCMS, which are
expensive and needs a lot of sample preparation work. These μPAD for heavy metal detection
involve complexion with ligands. Each metal has a colorimetric reagent which when it reacts
gives color. These reagents were placed in the hydrophilic zone and where left them to dry. To
identify metal, the paper is inserted into aqueous sample and metal ions containing the sample
react with the pre deposited reagent. Chen and co-workers determined Hg2+ using nanoparticles
based μPAD . Platinum nanoparticles catalysis TMB and it produces a blue product. The
presence of Hg2+ halts the oxidation process due to its interaction between the PtNPs and Hg2+,](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-8-320.jpg)
![9
for this reason the color signal intensity reduces and the intensity might be monitored with fiber
optic device. [3]
Nitrite, a common environmental pollutant can also be detected using μPAD sensors using
Griess reaction. Similarly NH3 and CO2 can be analyzed using μPAD. The μPAD is 2D paper
plat form that contains two layers of fabricated wax printed. One side is covered with Teflon tape
to prevent leakage. One layer of the paper contained NaOH solution and converts the ammonium
ions to NH3; a color is seen in the detection zone when the NH3 diffuses into the second layer,
because it reacts with bromothymol or nitro phenol which is immobilized in the paper. [7]
Sicard et al. reported inexpensive and portable μPAD sensor for on-site determination of
organophosphate pesticides. The mechanism of detection is change of colorless indoxyl acetate
into a product that is blue in color due to the enzyme catalyzed reaction. Different concentration
of pesticides gives different range of blue color. They also established and application installed
on smart phone that can interpret images.
Shingo and Karita carried out acid-base titration using μPAD . they also analyzed acidic hot
spring water. I. D. McKelvie and coworkers developed new onsite and low cost μPAD for the
determination of reactive phosphate in soil solution.[8]
Table: determining analytes using μPAD.
Target analyte
Fabrication
method
Detection method LOD Ref.
H2O2 Wax printing Colorimetric 0.65Mm [2]
Cd and Pb Wax printing Electrochemical 0.25 [9]
B-D-Glactoside Wax printing Fluorescence 0.7nm [10]
Uric acid Chemiluminescene 1.9mm [11]
Human breast
adenocarcinomcells
Wax printing Electrochemiluminscene 250cell/MI [12]
Penta chlorophenol
Wax screen
printing
Photoelecrochemical 4pg/MI [13]
Nitrite Stamping metho Colorimetric 5.6μm [7]
Phosphate Inkjet printing Colorimetric 1.6μm [7]
Mercury Wax printing Colorimetric 50nm [7]
Lead II Inkjet printing Colorimetric 0.05μm [7]
Cadmium II Paper cutting Electrochemical [7]
Chromium III wax printing Chemiluminescence 0.38 μm [7]
P-nitrophenol Wax printing Electrochemical 1.1 μm [7]
.Ph Wax printing Colorimetric [7]
H2S Wax printing Fluorescence 0.65 [7]
Copper Wax prining Colorimetric 64.8Mm [7]
Iron Wax printing Colorimetric 0.7 Mm [7]](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-9-320.jpg)
![10
3.2 Biomedical Applications.
Enzymatic reaction are common in biomedical applications of μPAD . Enzymes increase signal
because of their catalytic activity. They can be easily immobilized on the surface of paper and
creates colored products if substrate is present. High temperature experiments enzymes may
denature and this is disadvantages to the sensor. Important clinical biomarkers such as
creatinine, glucose and phenyl aniline can be detected using enzyme-based colorimetric μPAD .
[14] Has developed tear glucose analysis. Tear contains many types of biomolecules such
proteins, glucose and metal ions. Its glucose concentration range is from 0.1 to 0.6Mm. Mass
spectrometry and fluorescence analysis has shown significant glucose in tear fluid. μPAD using
blood as the biological fluid for the glucose measurement is invasive and discomfort for most
people because they need to use the needle several times during the day .to measure glucose
from the tear fluid mPAD is prepared by using wax printing and heating to melt the wax .
Adhesive tape is used for onside of the paper. TMB is used as the chromogenic solution in the
detection. Zone, followed by GOx and HRP.
Fig 6 μPAD used for glucose measurement](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-10-320.jpg)
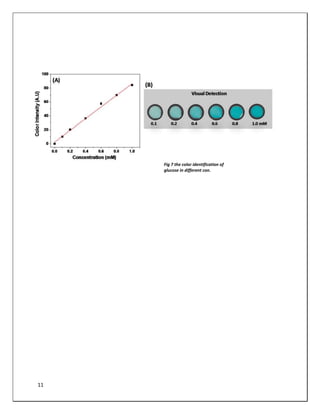

![13
Reference
[1] D. D. Liana, B. Raguse, J. Justin Gooding, and E. Chow, “Recent advances in paper-based
sensors,” Sensors (Switzerland), vol. 12, no. 9, pp. 11505–11526, 2012.
[2] Y. He, Y. Wu, J. Z. Fu, and W. Bin Wu, “Fabrication of paper-based microfluidic analysis
devices: a review,” RSC Adv., vol. 5, no. 95, pp. 78109–78127, 2015.
[3] Y. Yang, E. Noviana, M. P. Nguyen, B. J. Geiss, D. S. Dandy, and C. S. Henry, “Paper-
Based Microfluidic Devices: Emerging Themes and Applications,” Anal. Chem., vol. 89,
no. 1, pp. 71–91, 2017.
[4] D. M. Cate, J. A. Adkins, J. Mettakoonpitak, and C. S. Henry, “Recent Developments in
Paper-Based Microfluidic Devices,” Anal. Chem., vol. 87, no. 1, pp. 19–41, 2015.
[5] Y. Xia, J. Si, and Z. Li, “Fabrication techniques for microfluidic paper-based analytical
devices and their applications for biological testing: A review,” Biosens. Bioelectron., vol.
77, pp. 774–789, 2016.
[6] T. Akyazi, L. Basabe-Desmonts, and F. Benito-Lopez, “Review on microfluidic paper-
based analytical devices towards commercialisation,” Anal. Chim. Acta, vol. 1001, pp. 1–
17, 2018.
[7] M. I. G. S. Almeida, B. M. Jayawardane, S. D. Kolev, and I. D. McKelvie,
“Developments of microfluidic paper-based analytical devices (μPADs) for water
analysis: A review,” Talanta, vol. 177, no. August 2017, pp. 176–190, 2018.
[8] S. Karita and T. Kaneta, “Acid-base titrations using microfluidic paper-based analytical
devices,” Anal. Chem., vol. 86, no. 24, pp. 12108–12114, 2014.
[9] P. Rattanarat, W. Dungchai, D. Cate, J. Volckens, O. Chailapakul, and C. S. Henry,
“Multilayer paper-based device for colorimetric and electrochemical quantification of
metals,” Anal. Chem., vol. 86, no. 7, pp. 3555–3562, 2014.
[10] N. K. Thom, G. G. Lewis, K. Yeung, and S. T. Phillips, “Quantitative fluorescence assays
using a self-powered paper-based microfluidic device and a camera-equipped cellular
phone,” RSC Adv., vol. 4, no. 3, pp. 1334–1340, 2014.
[11] J. Yu, S. Wang, L. Ge, and S. Ge, “A novel chemiluminescence paper microfluidic
biosensor based on enzymatic reaction for uric acid determination,” Biosens. Bioelectron.,
vol. 26, no. 7, pp. 3284–3289, 2011.
[12] L. Wu et al., “Paper-based electrochemiluminescence origami cyto-device for multiple
cancer cells detection using porous AuPd alloy as catalytically promoted nanolabels,”
Biosens. Bioelectron., vol. 63, pp. 450–457, 2015.
[13] G. Sun, P. Wang, S. Ge, L. Ge, J. Yu, and M. Yan, “Photoelectrochemical sensor for
pentachlorophenol on microfluidic paper-based analytical device based on the molecular
imprinting technique,” Biosens. Bioelectron., vol. 56, pp. 97–103, 2014.](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-13-320.jpg)
![14
[14] E. F. M. Gabriel, P. T. Garcia, F. M. Lopes, and W. K. T. Coltro, “Paper-based
colorimetric biosensor for tear glucose measurements,” Micromachines, vol. 8, no. 4, pp.
1–9, 2017.](https://image.slidesharecdn.com/paperbasedanalyticaldevises-190211161021/85/Paper-based-analytical-devises-14-320.jpg)