This document summarizes a study that utilized waste from carbide lime production to synthesize precipitated calcium carbonate (PCC). The researchers found that carbide lime waste, which contains calcium hydroxide, can be used to produce milk of lime for PCC precipitation when bubbling carbon dioxide gas through it. They investigated how different reaction parameters like milk of lime concentration, carbon dioxide flow rate, and reaction time influence the purity, morphology, and particle size of the synthesized PCC. Under optimized conditions of 2M milk of lime concentration, 90 minute reaction time, and 452.30 mL/min carbon dioxide flow rate, they were able to produce high purity PCC (99%) with a desirable scalenohedron

![Recent advances in the utilization of carbide lime have
focused on PCC production because of its humongous
applications. As long as PCC meets certain purity
requirement, it can be utilized as an artificial pigment in
paper, plastics, drug carriers in pharmaceuticals, sealants,
food industries, paint manufacturing and fillers in adhe-
sives (Nasser et al. 2015; Thenepalli et al. 2015). As a filler
material, it provides high tensile reinforcement due to its
unique particle size and morphology (Sae-oui et al. 2009).
PCC is usually produced via three routes, namely the cal-
cium hydroxide–sodium hydroxide, calcium chloride–
sodium carbonate double salt decomposition process, and
carbonization process (Onimisi et al. 2016). All these
routes utilize milk of lime usually generated from lime-
stone. The limestone is first calcined (*1000 °C) to form
CaO. Thereafter, the obtained lime is screened to remove
impurities in the limestone and the lime is dispersed in
water to form milk of lime. Finally, CO-2
gas is passed in
the milk of lime resulting in calcium carbonate precipita-
tion. Therefore, the CO2 produced during the calcium
carbide production can as well be channeled to produce
PCC. This will minimize or compensate for the high
energy input during calcium carbide production. The car-
bonation process enables PCC of a given specification to be
produced in a dedicated plant, irrespective of the local
geology. The fineness of the particles, as well as the crystal
morphology (e.g., aragonite, calcite), is controlled by
temperature, concentration of reactants and time. The
process is environment-friendly and does not emanate toxic
pollutants (Suwanthai et al. 2015).
As a continuous effort to utilize waste materials gener-
ated from industries, researchers have produced PCC using
varying wastes from gypsum and steel slags (Mattila et al.
2012). Ciullo (1996), reported that high purity PCC
([95 %) can be produced using pseudo-catalytic lixiviant
to selectively extract calcium from slag material before
being dissolved as PCC. Also, ammonium salts have been
used to selectively extract calcium from steel slag, result-
ing in high purity PCC product and reduction in CO2
emission (Adams 2005; De Crom et al. 2015). They
reported that the smallest solid to liquid ratio 5 g/L resulted
in the maximum calcium extraction efficiency (73 %),
while the reverse using 100 g/L produce the lowest
extraction efficiency of 6 %. Liu et al. (2016), investigated
the performance of two organic acids (succinic and acetic
acid) for the possible extraction of calcium from steel-
making slag for PCC production. They observed that the
carbonation of succinic acid leachate did not result in the
production of PCC, while the carbonation of acetic acid
leachate resulted in the synthesis of PCC. Furthermore,
studies by Suwanthai et al. (2015) hinted that high purity
PCC (mainly calcite) can be synthesized from gypsum
waste by using an acid gas (H2S) to improve the aqueous
dissolution of the poorly soluble CaS. Huang et al. (2007)
produced high purity PCC (mainly amorphous) from
medium and low-grade limestone using strongly acidic
cation exchange resin. This improvement was due to the
reaction of HCO3
-
in aqueous solution during the slaking
reaction process. Valuable information from their study
indicated that impurities were eliminated during the car-
bonation reaction.
The use of carbide lime waste for the synthesis of PCC
has not been adequately reported. Therefore, the appro-
priate techniques, operating conditions and physical prop-
erties of carbide lime sludge in PCC production were
envisaged for the first time in this study. Also, the influ-
encing parameters that control the precipitation process,
products morphology and particle size were thoroughly
investigated.
Experimental methodology
Material
In order to carry out this experiment, calcium carbide
produced by MCB industries Sdn. Bhd (Malaysian Carbide
Berhad), Kemunting, Taiping was used. The typical
chemical composition [by X-ray fluorescence (XRF)] of
the CaC2 is tabulated in Table 1. Pure CO2 gas was sup-
plied by Merck. Conductivity meter-Istek 455C model for
pH, reaction temperature, conductivity and total dissolved
solid (TDS) online monitoring.
Hydrated milk of lime precipitation
Hundred grams calcium carbide was dissolved in 1 L
double distilled water, resulting in acetylene gas liberation
and precipitation of hydrated lime (Ca(OH)2). When the
reaction is complete, observed from high pH [12, the
resultant hydrated lime is then dried in an oven at 105 °C
for 8 h, and subsequently screened to obtain 125 lm
powder. In this investigation, various concentration of milk
of lime was prepared from the carbide lime powder and
experimented in accordance with operating variables as
designed in Table 2. Milk of lime suspension produced by
dissolution of dried carbide lime powder was screened to
remove any coarse grits that may affect PCC particle
morphology.
Precipitated calcium carbonate production
The precipitation of calcium carbonate production using
CO2–Ca(OH)2 was carried out in an agitated reaction
vessel (a 1000 mL rounded glass with a multi-socket
reaction vessel). CO2 gas was bubbled through the batch
1251 Page 2 of 7 Environ Earth Sci (2016)75:1251
123](https://image.slidesharecdn.com/89ab2e96-62dc-4ca4-8f76-1b5ddc9fb580-160919040223/85/Paper-2-2-320.jpg)
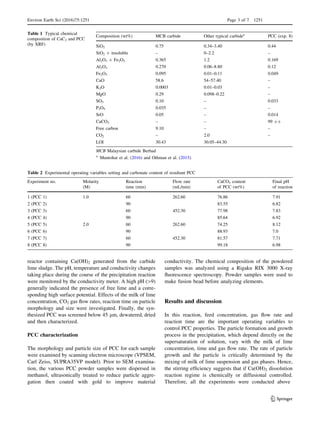

![reaction times (60 and 90 min) increased marginally
(Table 2) as CO2 flow rate increased (262–452 mL/min).
However, different crystals morphology was observed at
different reaction times which are attributed to increasing
CO2 flow rate. The PCC-3 (Fig. 1c) produced at similar
conditions as PCC-1, displayed a cubic-like rhombohedron
crystal morphology particle. This can be explained by the
presence of lower ionic strength or OH-
of the Ca(OH)2
suspension with higher a presence of CO2 gas, promoting
the yield of rhombohedron crystals. Similarly, PCC-4
produced at same conditions [1 M Ca(OH)2 and 90 min]
and different CO2 flowrate as PCC-2 presented a closely
packed cluster of dendritic-like (radiated) scalenohedral
PCC particles, prismatic to needle-like crystals (Fig. 1d).
In general, increasing the bubbling rate not only reduced
the particle size but also resulted in a change in crystal
morphology. For the higher reactant concentration (2 M
Ca(OH)2), boosting the rate of CO2 flow triggered gener-
ation of finer PCC powder. In spite of that, PCC-6 (Fig. 2f),
with a good scalenohedral crystal was attained, increasing
the flow rate and bubbling time has significantly reverted
the crystal shape again into cubic-like, rhombohedron PCC
(Fig. 2h, PCC-8).
Temperature–conductivity relationship
Conductivity sensors are usually used to measure the
concentration of total dissolved solids (TDS) during PCC
production. The conductivity of acid or base depends on
both ion concentration and ion mobility. The ion mobility
is promoted by temperature which increases about 2 % for
each °C increase in temperature. The degree at which
temperature affects conductivity depends on the types of
ions involved. The gradual temperature increase, and
decreasing conductivity and TDS at precipitation reaction
regime are presented in Figs. 3, 4 and 5. From Fig. 3, the
conductivity for 8 PCC experiments decreases steadily
with increasing precipitation time. Similarly, the TDS
decreases as the conductivity decreases (Fig. 4). Since the
pH is a measure of hydrogen ion concentration, hence,
lower pH (i.e., higher H?
ion concentration) translates to
higher conductivity. It is crucial to end the precipitation
reaction at optimum pH where efficient and effective
conversion of milk of lime to CaCO3 is achieved and
before the CO2 concentration becomes too high (increased
acidity) to initiate dissolution of the suspended CaCO3
precipitate. The initial pH of the milk of lime is greater
PCC-5 PCC-6
PCC-7 PCC-8
Fig. 2 Images of the synthesized PCC (PCC-5 to PCC-8) produced at a reactant concentration of 2 M
Environ Earth Sci (2016)75:1251 Page 5 of 7 1251
123](https://image.slidesharecdn.com/89ab2e96-62dc-4ca4-8f76-1b5ddc9fb580-160919040223/85/Paper-2-5-320.jpg)