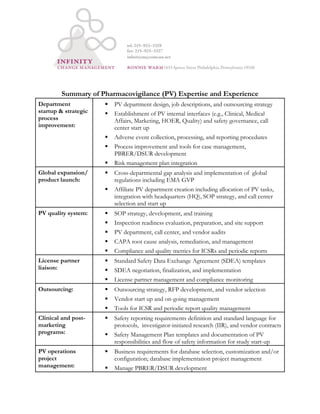
This document summarizes expertise in pharmacovigilance (PV) including:
1) Establishing PV departments, procedures for adverse event reporting, and process improvements.
2) Expanding globally and launching new products, including creating affiliate PV departments and ensuring compliance.
3) Managing quality systems with SOPs, audits, compliance, and issue remediation.