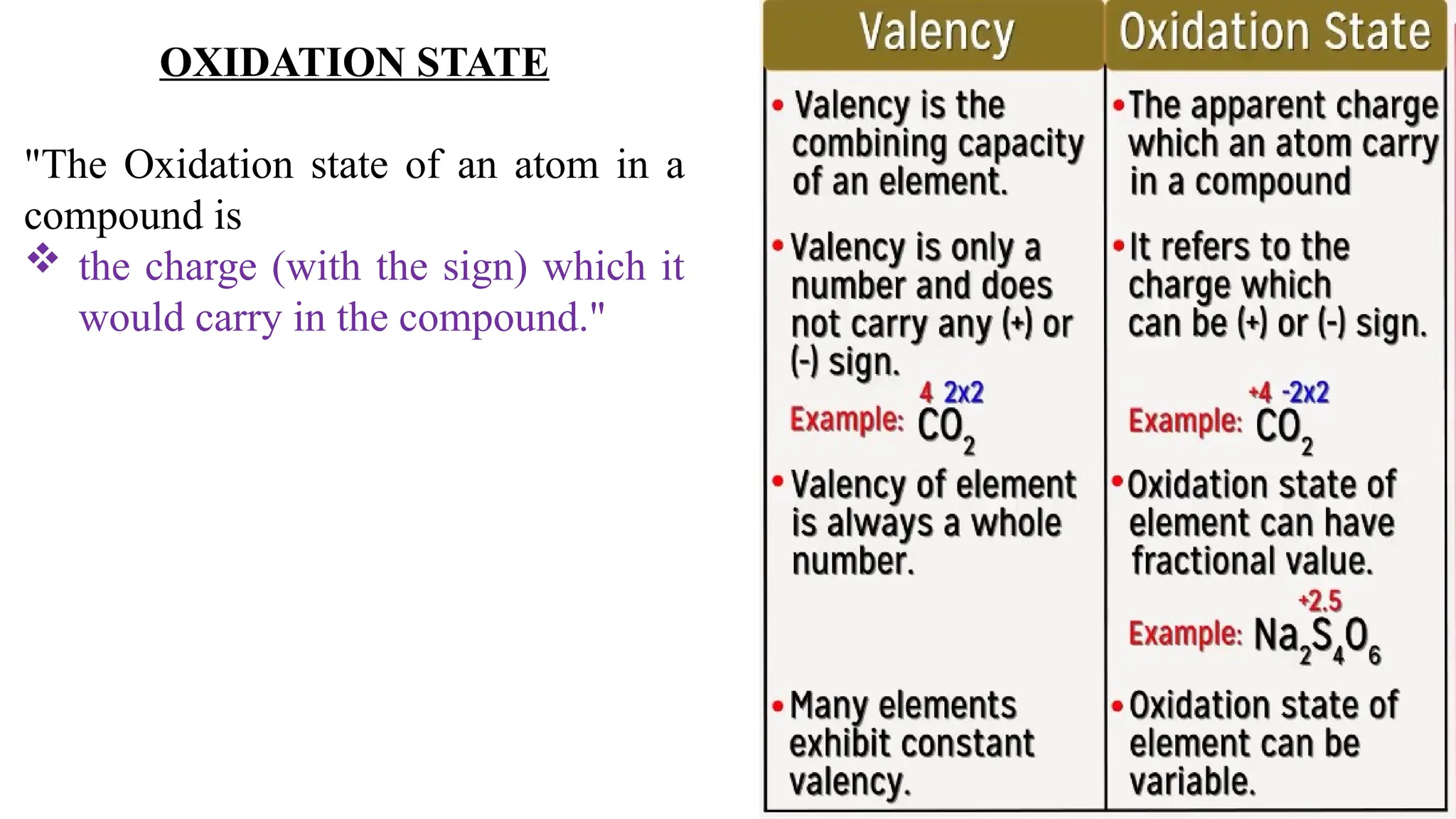
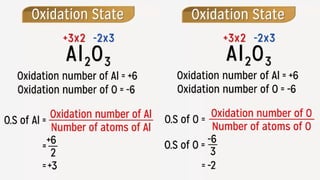
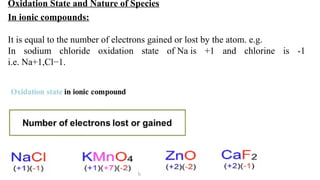
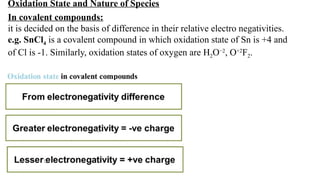
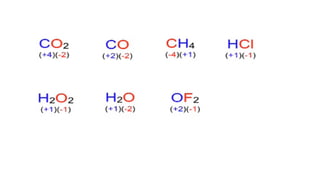
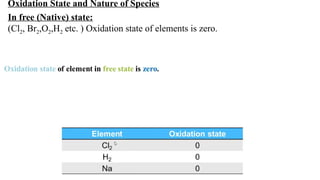
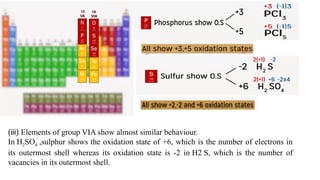
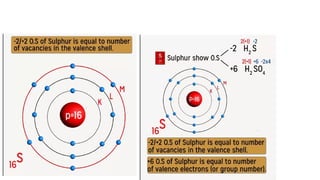
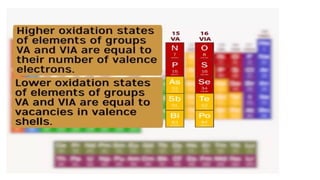
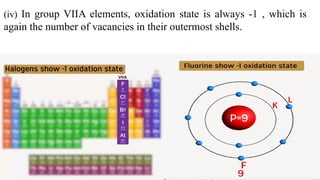
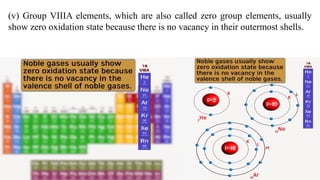
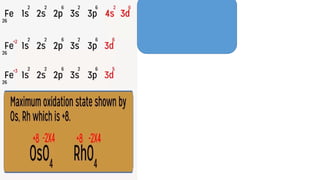
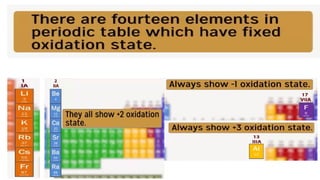
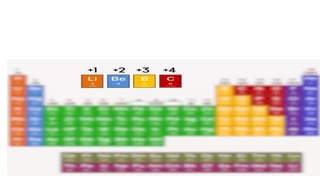
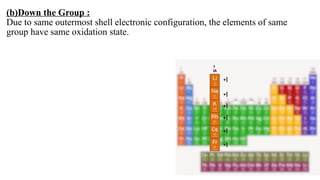
The document explains oxidation states of atoms in compounds, how they relate to ionic and covalent bonds, and how they are influenced by the group's position in the periodic table. It details the oxidation states for various groups, emphasizing that typical elements show oxidation states correlated to their group numbers, while transition elements exhibit multiple oxidation states due to partially filled d-orbitals. The document also discusses oxidation states in free states and provides examples across groups of the periodic table.