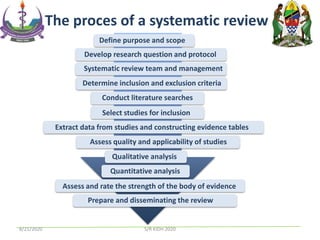
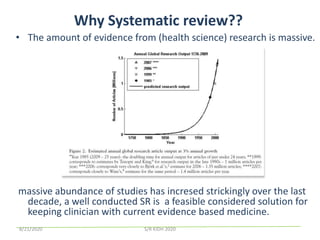
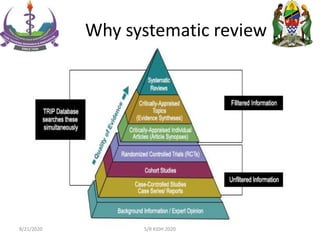
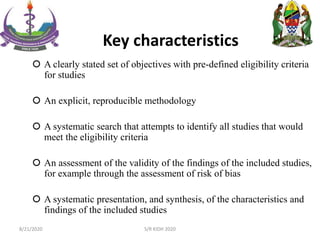
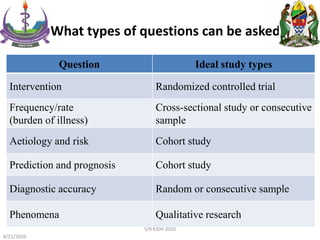
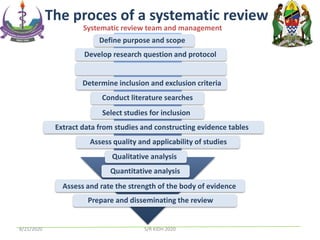
The document provides an overview of the steps involved in conducting a systematic review, including forming a team, developing a search strategy and inclusion/exclusion criteria, searching databases, extracting and analyzing data, and reporting results. Key aspects of systematic reviews discussed include developing a protocol, conducting a comprehensive literature search, assessing risk of bias, and synthesizing evidence both qualitatively and quantitatively through meta-analysis when possible. Challenges of systematic reviews include the time and resources required as well as ensuring methodological rigor.










![Search strategy
Search ((cholera) AND (((waves) OR
("lineage"[All Fields])) OR (biotypes))) AND
(((((((("east africa"[All Fields]) OR
("tanzania"[All Fields])) OR ("rwanda"[All
Fields])) OR ("south sudan"[All Fields])) OR
("kenya"[All Fields])) OR ("uganda"[All
Fields])) OR (burundi)) OR (zanzibar))
8/21/2020 S/R KIDH 2020](https://image.slidesharecdn.com/overviewonsystematicreviewma-kidh-200821074658/85/Over-view-on-systematic-review-17-320.jpg)