Embed presentation
Download to read offline

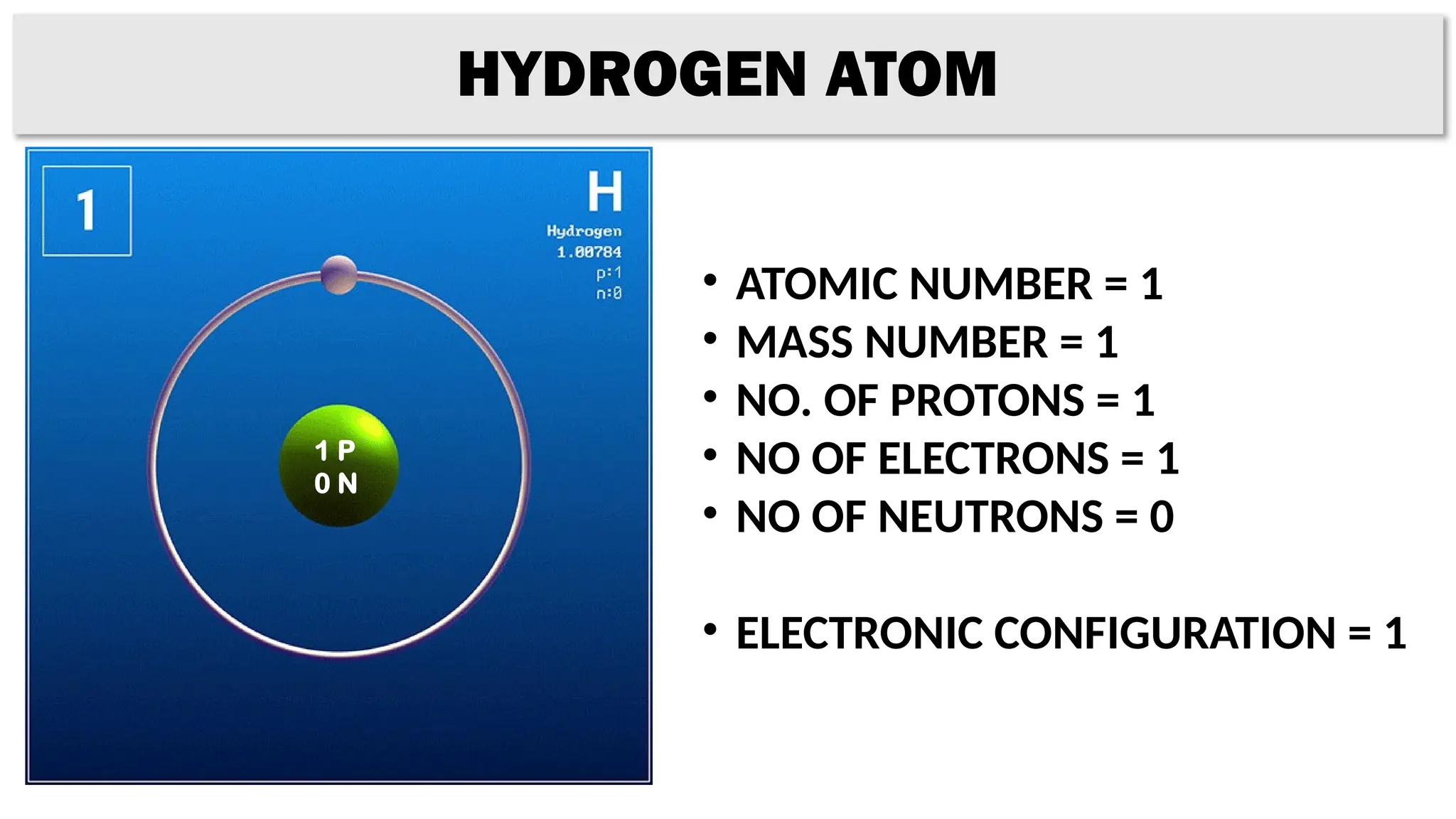
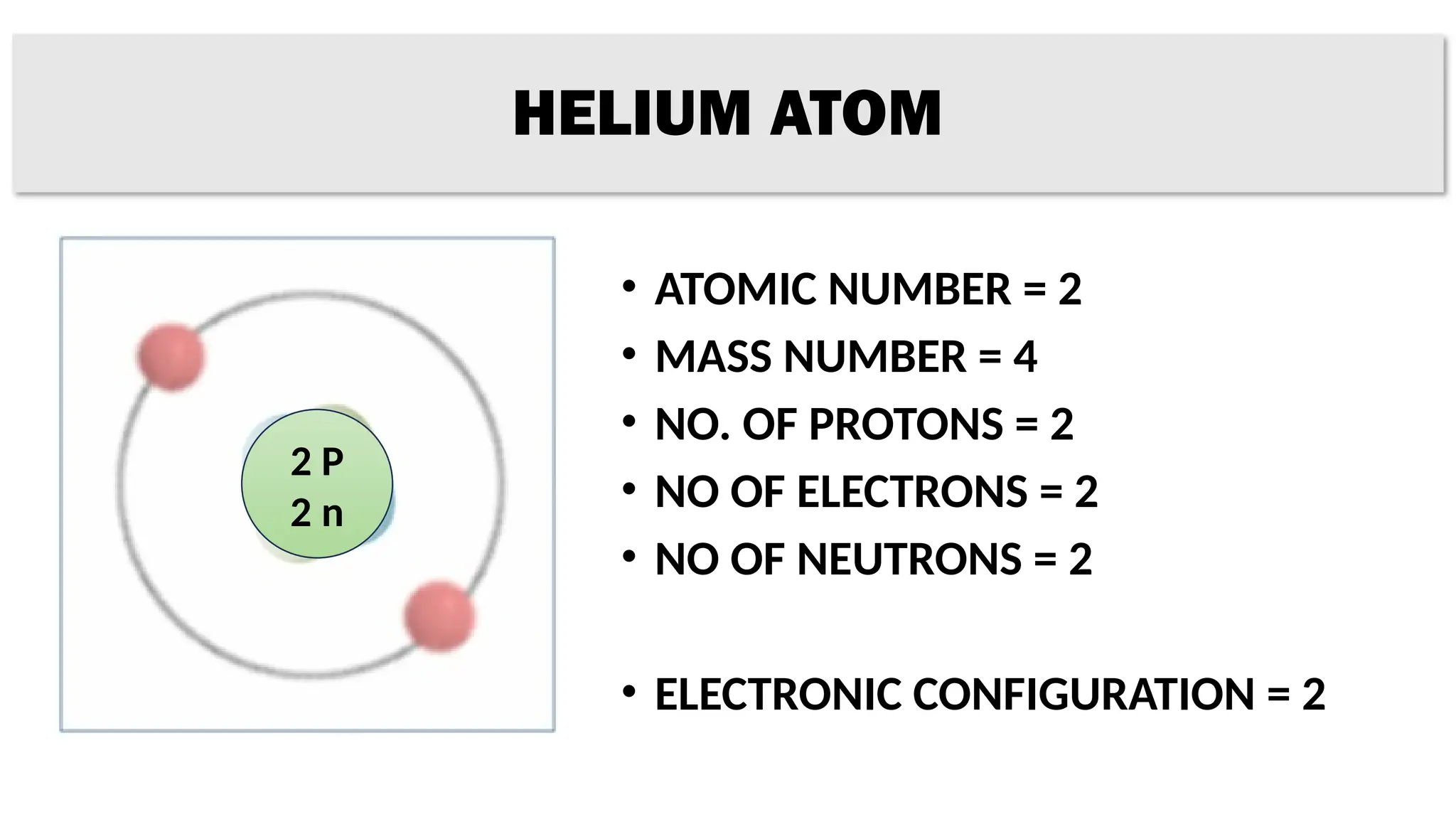
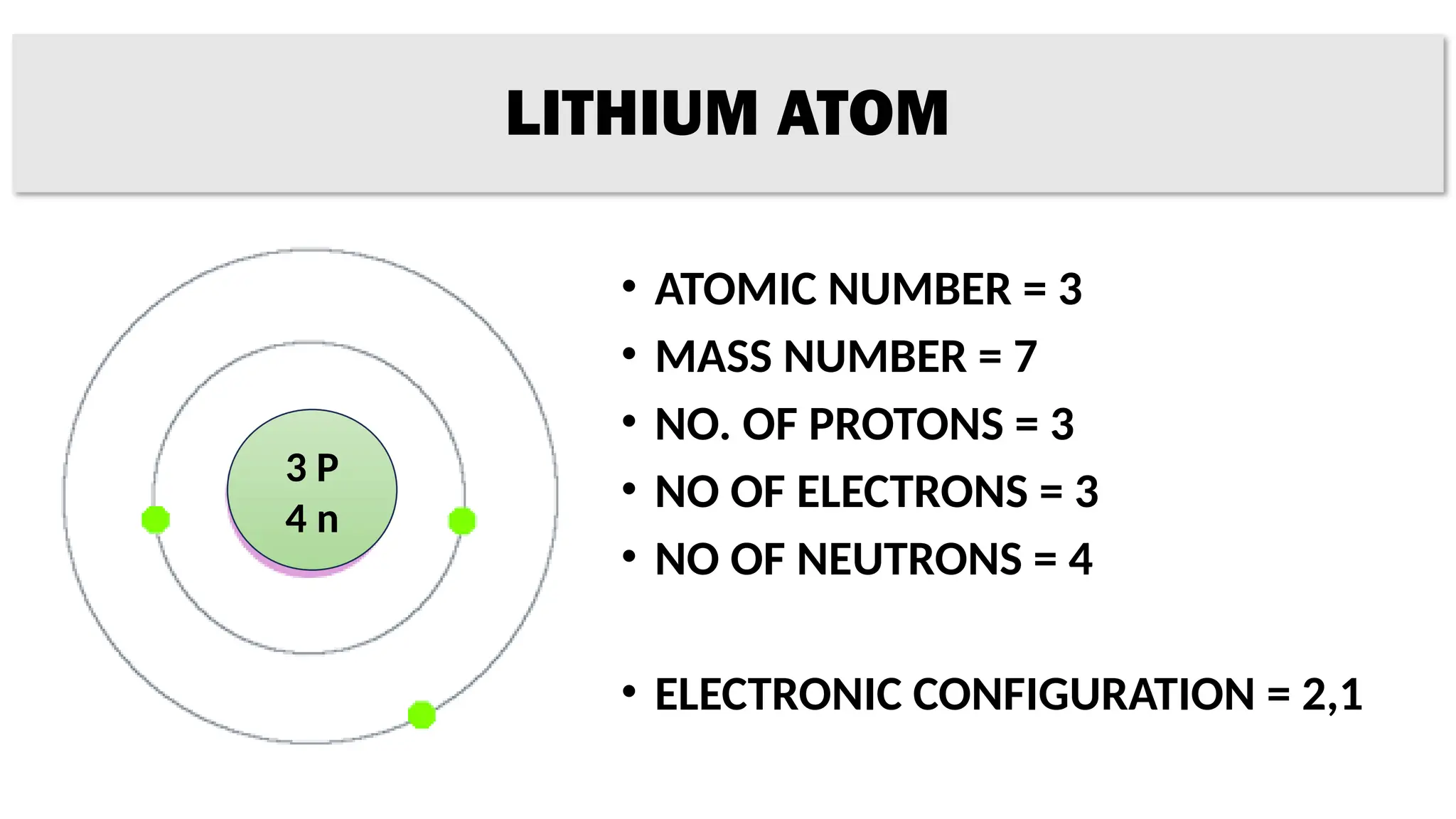
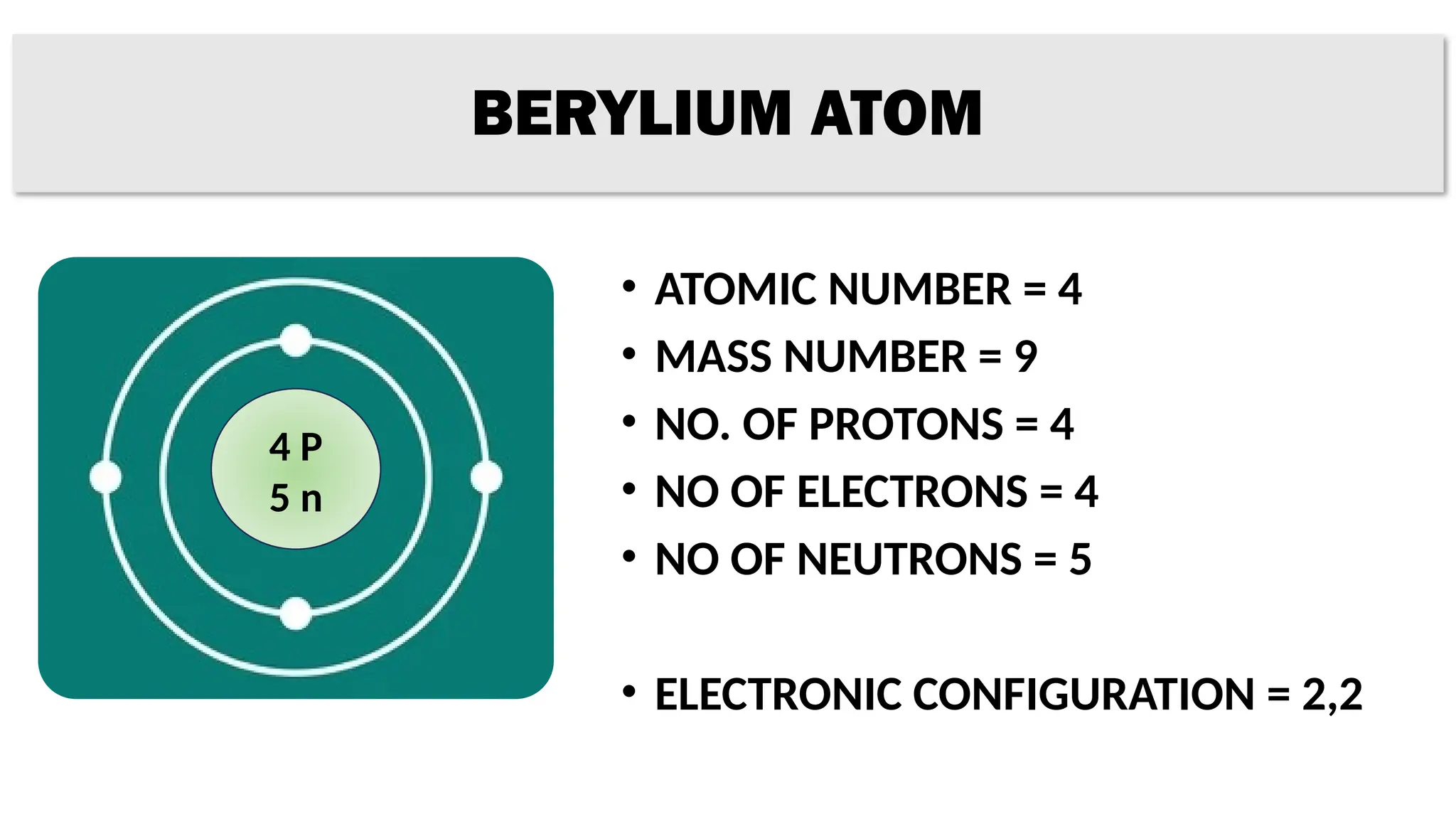
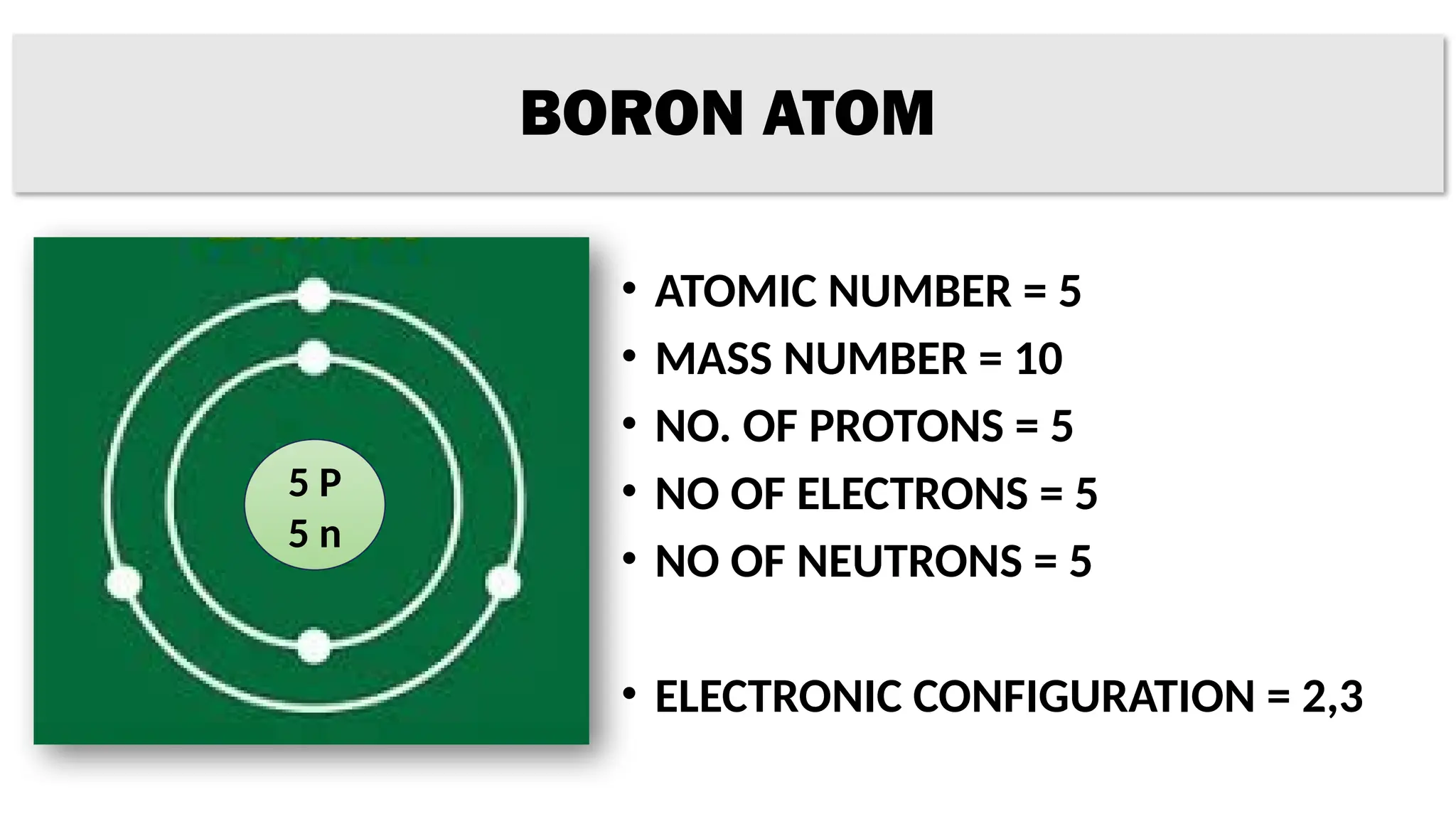
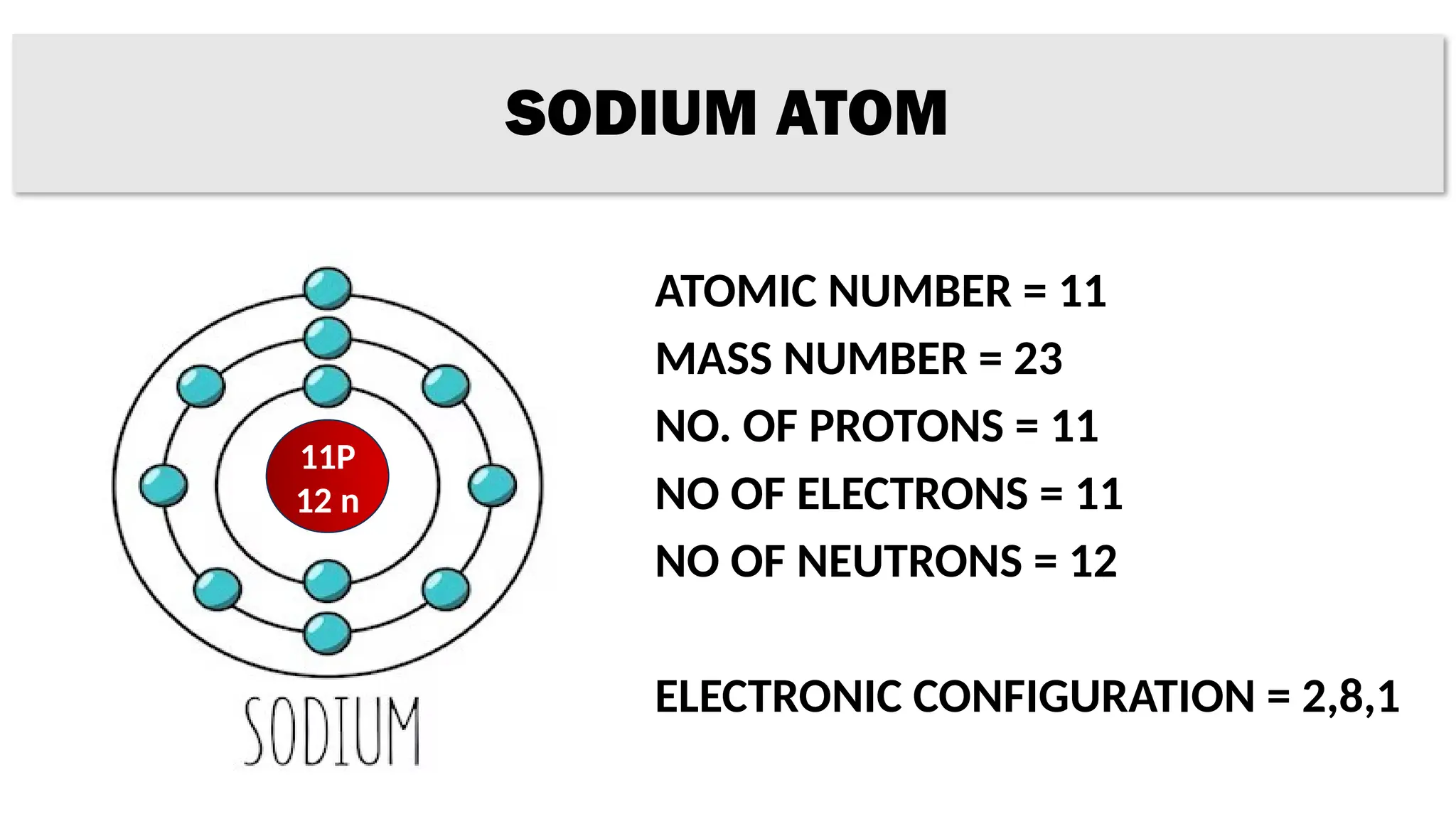
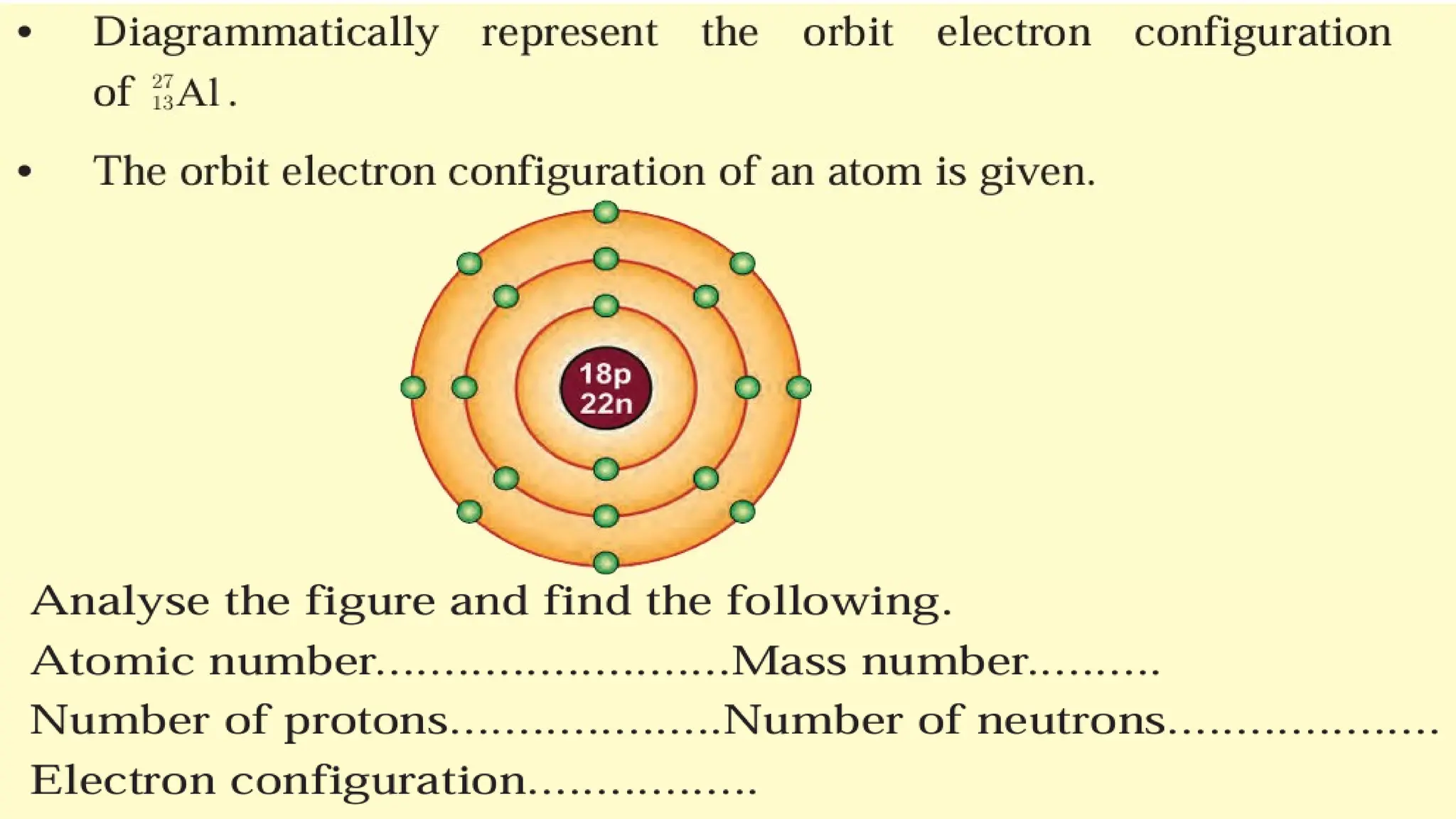

The document provides details on the structure of various atoms, including hydrogen, helium, lithium, beryllium, boron, and sodium. For each element, it lists attributes such as atomic number, mass number, number of protons, electrons, neutrons, and their electronic configuration. This information summarizes the basic atomic structure and properties of these elements.









