This document dwells into the Various methods used for optimization of fermentation process and their applications in food industry.
Fermentation optimization is a cornerstone of efficient and scalable bioproduction. It
involves meticulously fine-tuning various process parameters to create an ideal environment
for the target microorganisms. This translates to maximizing desired outputs, such as product
titer and yield, while ensuring process robustness and cost-effectiveness. The ultimate goal is
to create an optimal environment for the target microorganisms, allowing them to thrive and
produce the target molecule at the highest possible rate and concentration (titre) while
minimizing production costs and ensuring consistent product quality. This optimization process
is crucial across various industries that rely on fermentation, including: Optimizing
fermentation for beer, yogurt, or cheese production can enhance flavour profiles, improve
texture, and increase yield, Fine-tuning fermentation processes for bioethanol or biodiesel can
significantly impact production efficiency and fuel quality and optimizing fermentation for
antibiotics or other drugs can maximize yield, reduce production time, and ensure consistent
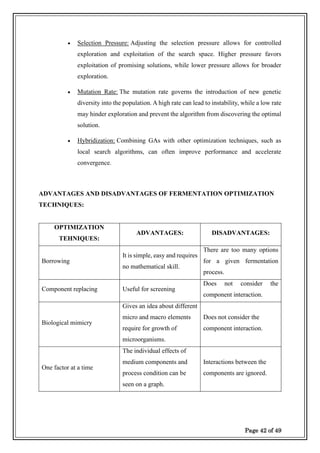
drug potency. Many optimization techniques are available for optimization of fermentation
medium and fermentation process conditions such as
✓One-factor-at-a-time.
✓ Borrowing.
✓ Component replacing.
✓ Biological Mimicry
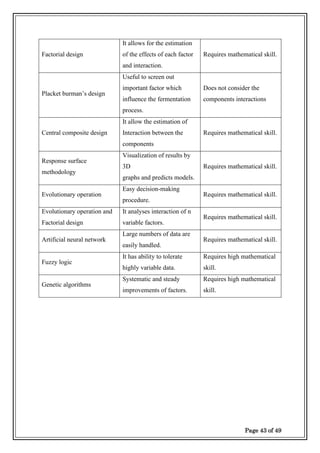
✓ Factorial Design.
✓ Placket and Burmar’s Design.
✓ Central composite Design.
✓ Response Surface methodology.
✓ Evolutionary operation.
✓ Evolutionary operation factorial design.
✓ Artificial neural network.
✓ Fuzzy logic.
✓ Genetic Algorithms
Each
optimization technique has its own advantages and disadvantages.