Embed presentation
Downloaded 132 times




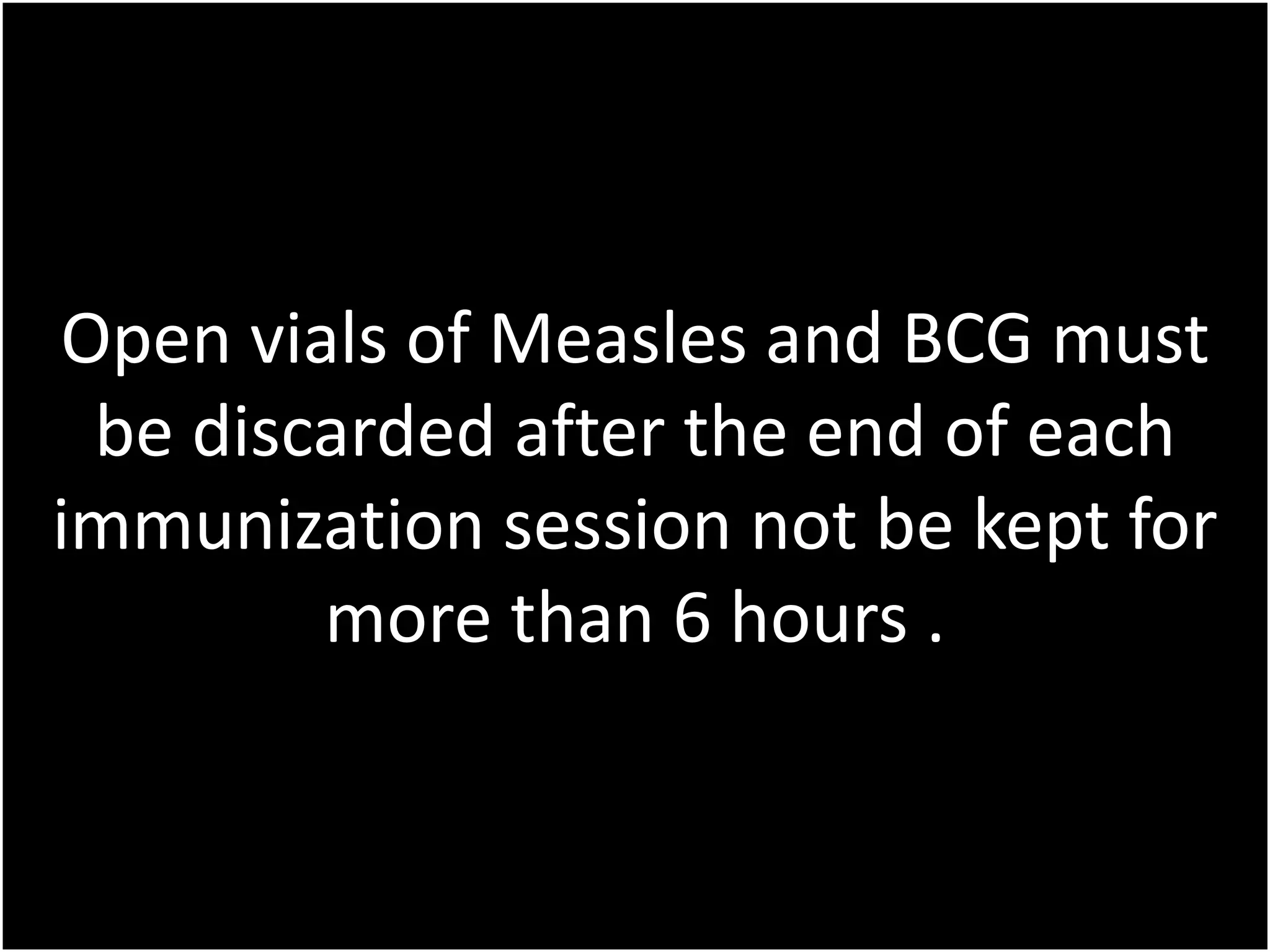




A policy on managing opened vaccine vials is needed to minimize waste while ensuring vaccine effectiveness. Multi-dose vials of certain vaccines like OPV, DTP, and hepatitis B can be used over multiple immunization sessions for up to 4 weeks if stored properly and have not expired or been contaminated. Open vials of measles and BCG vaccines must be discarded after each session or within 6 hours. All opened vials must be discarded immediately if aseptic procedures were not followed or the vial is suspected of being contaminated.







