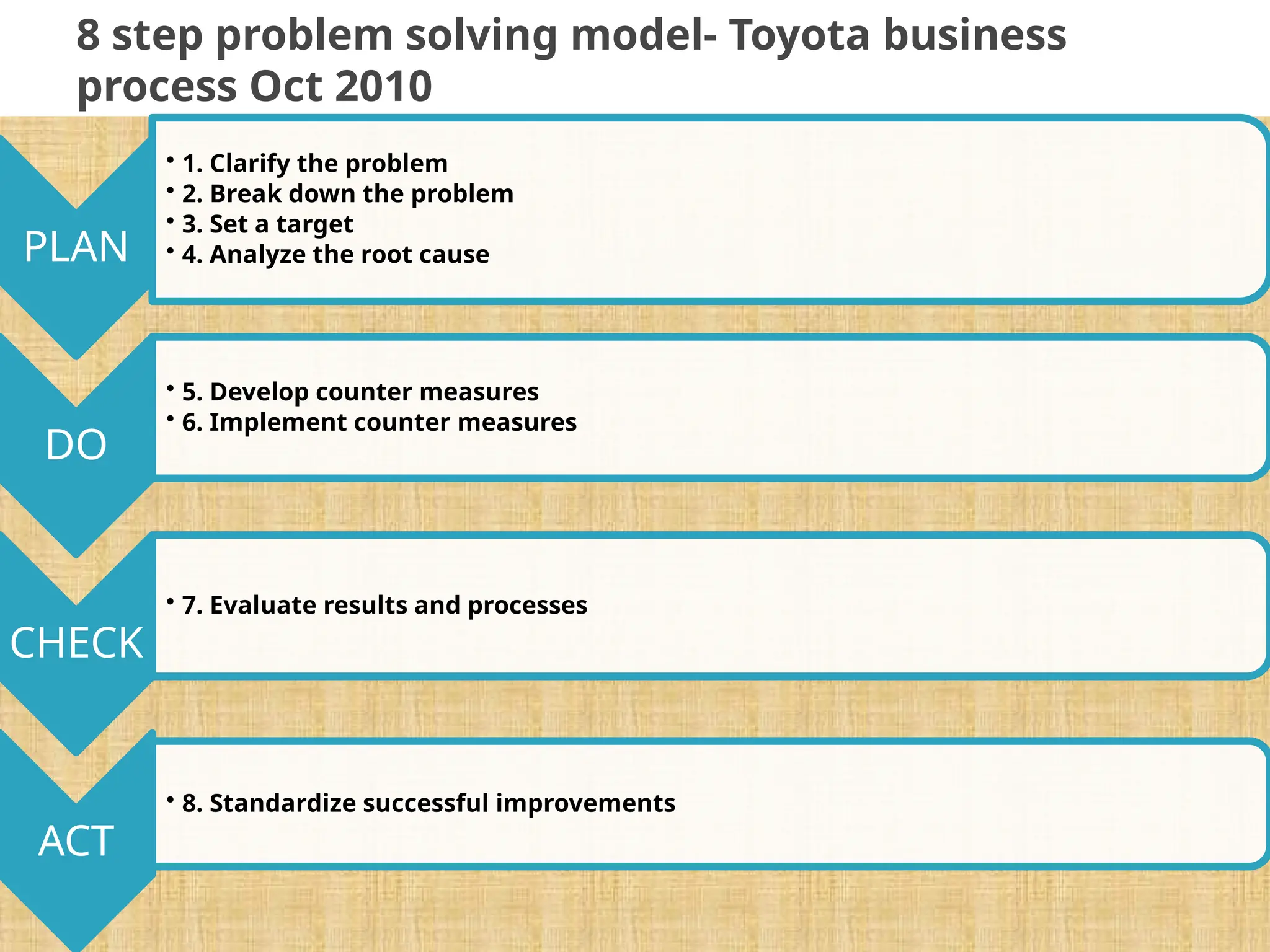
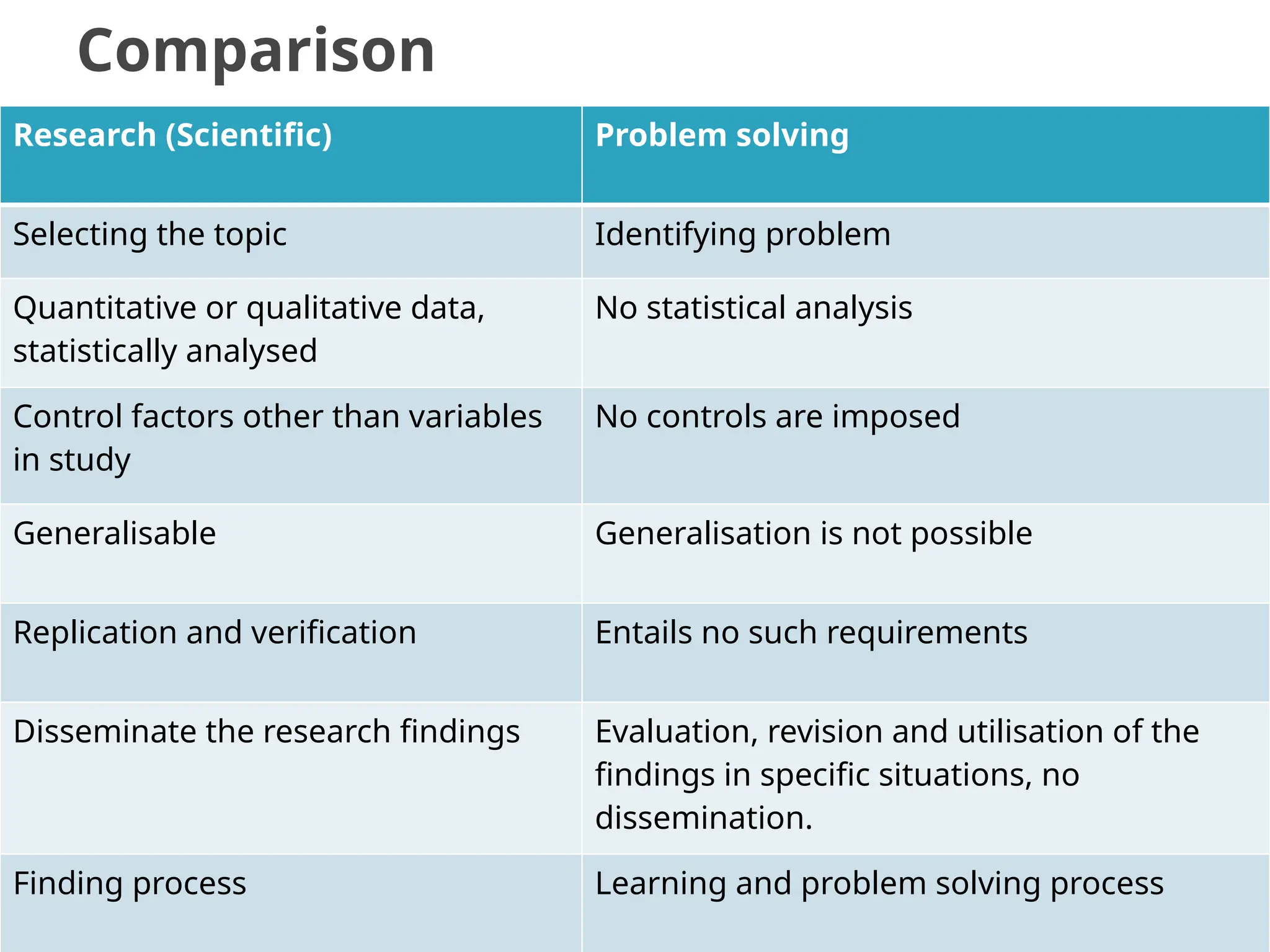
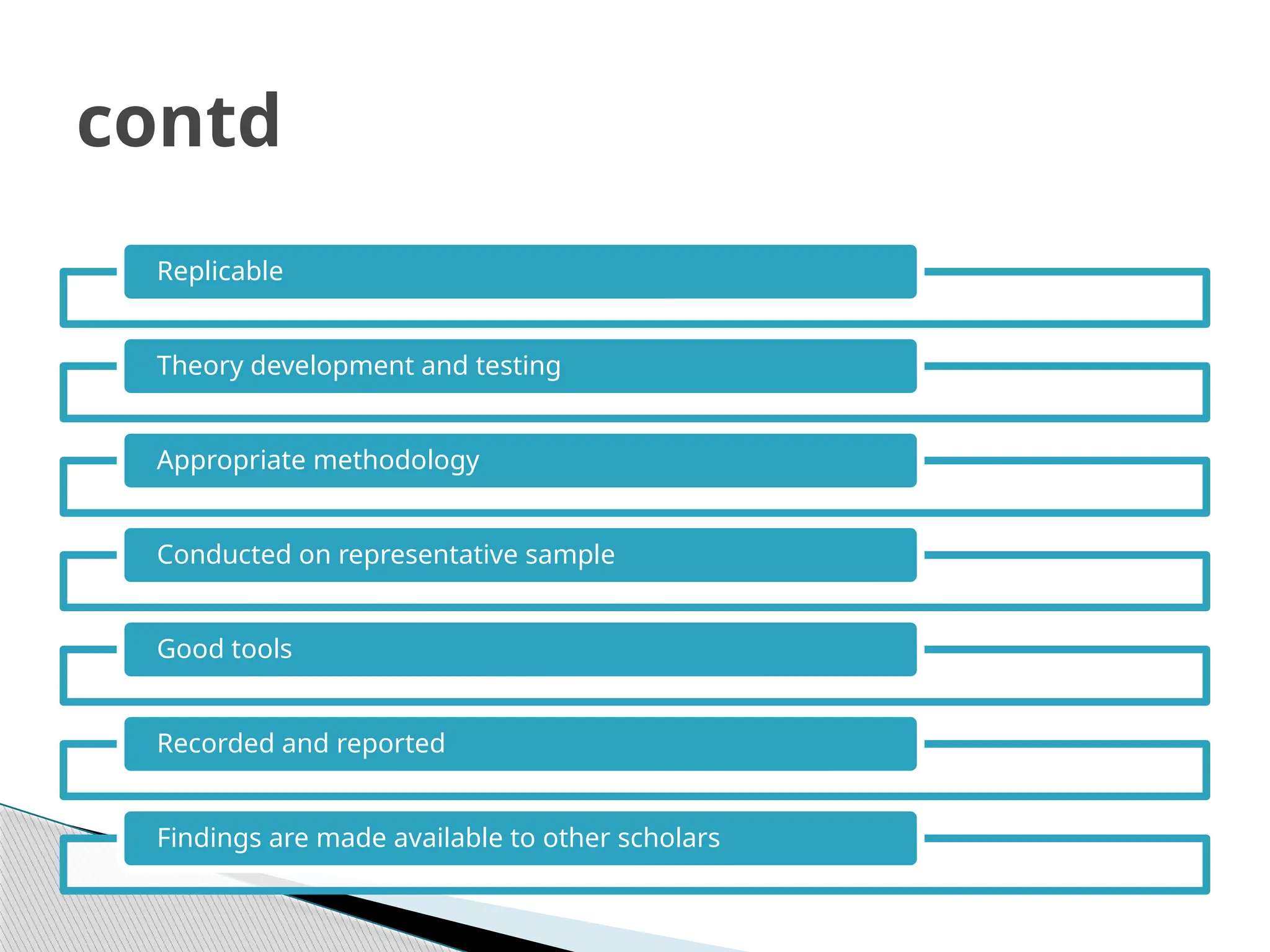
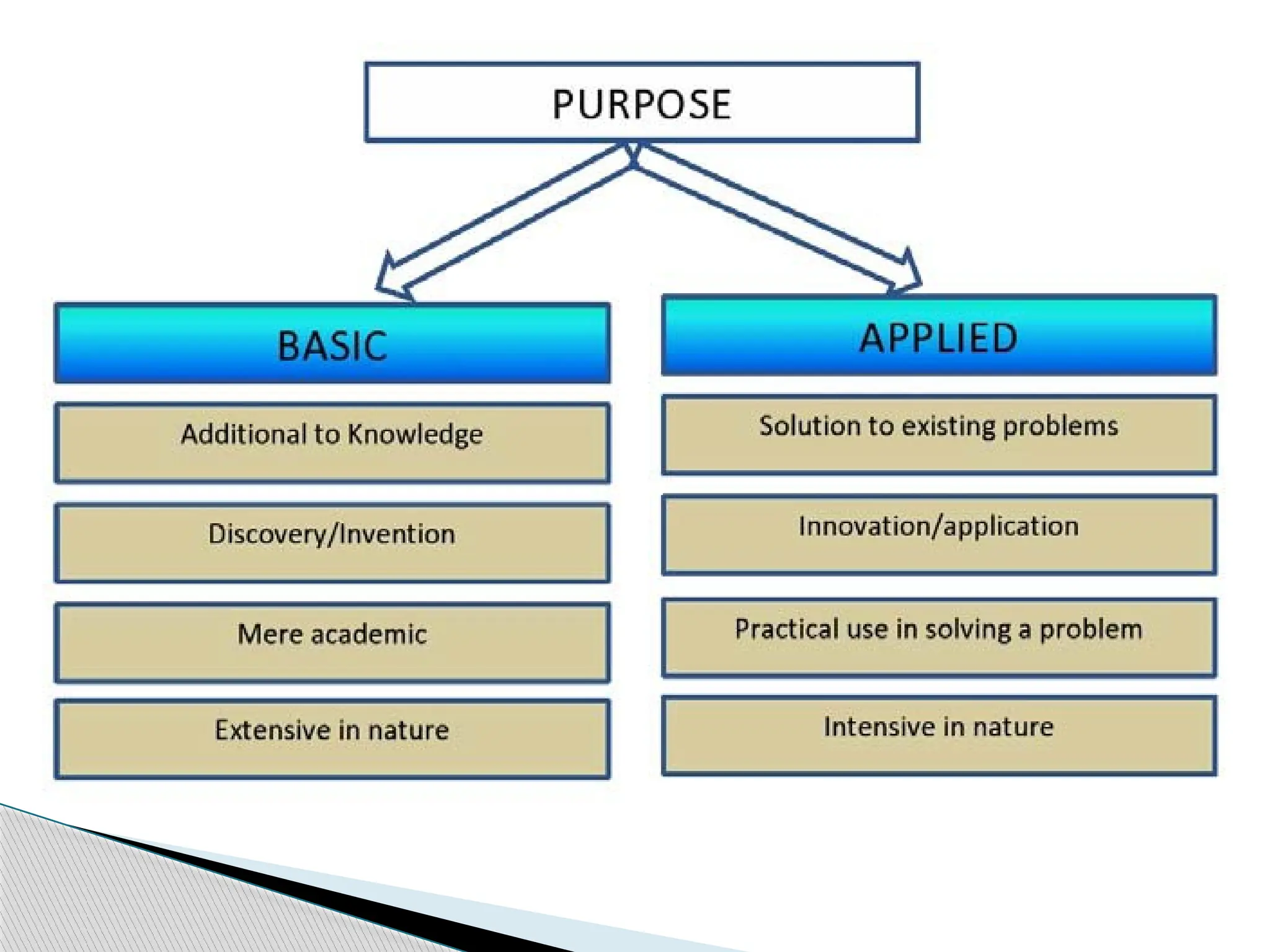
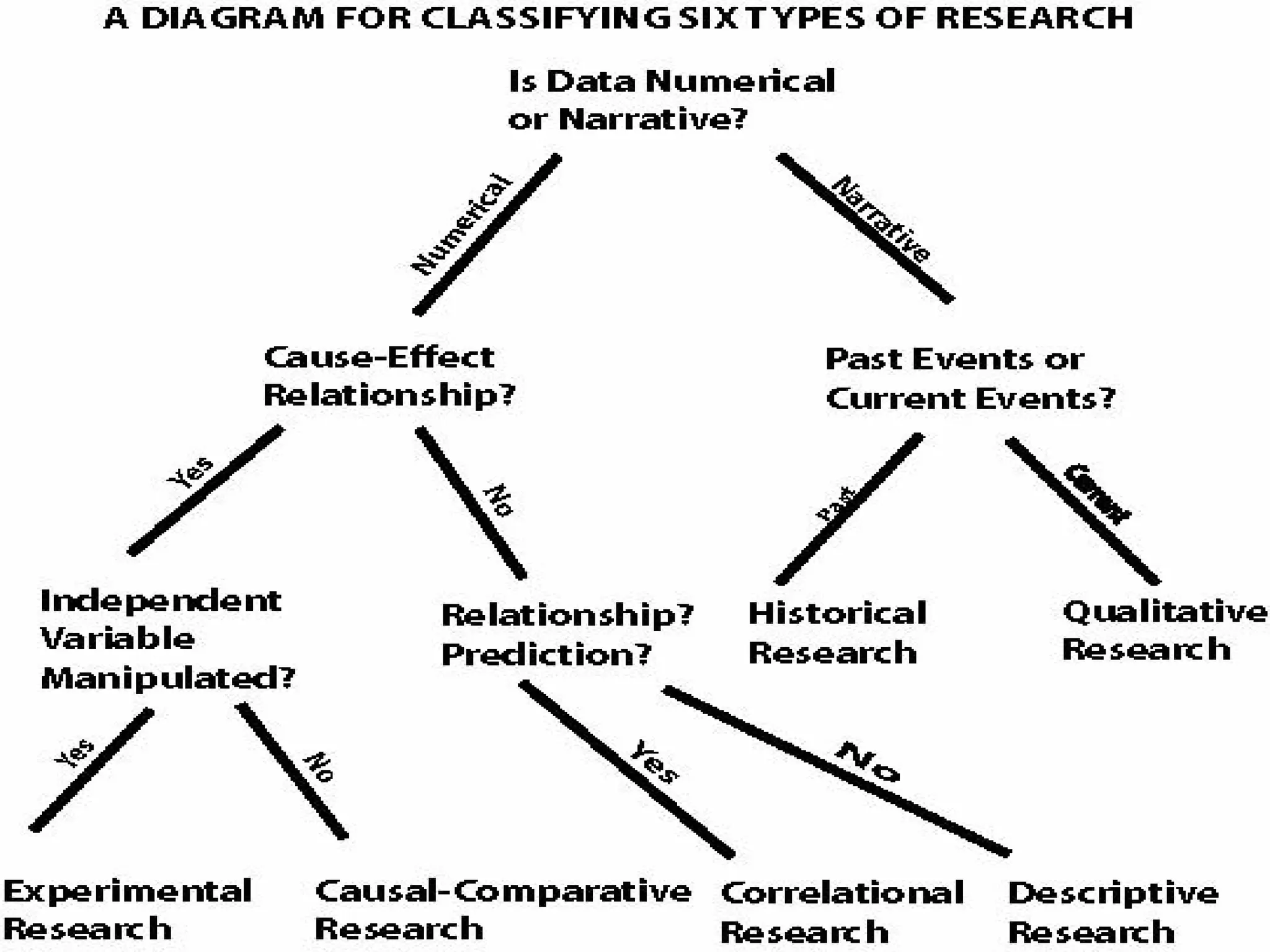
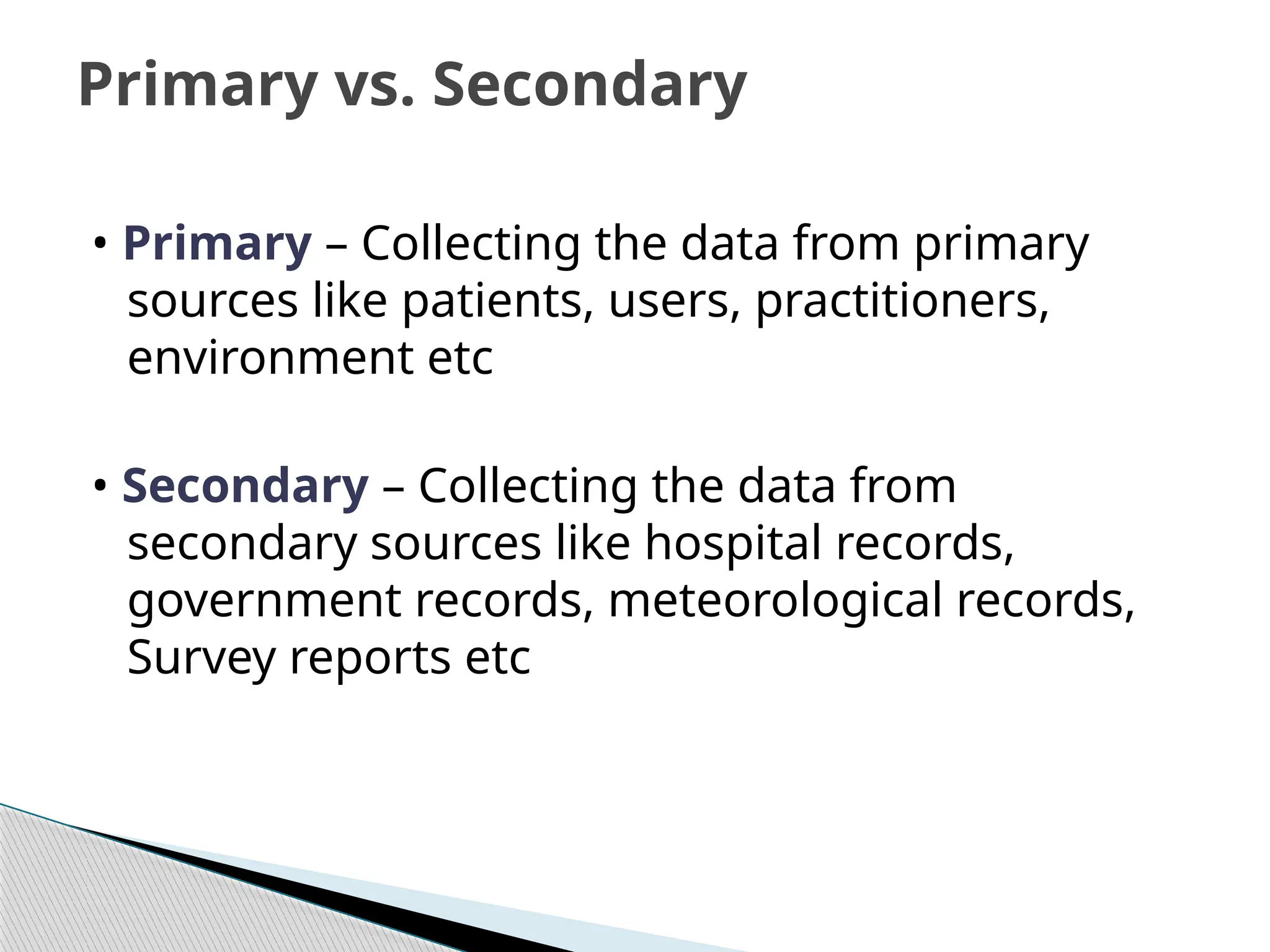
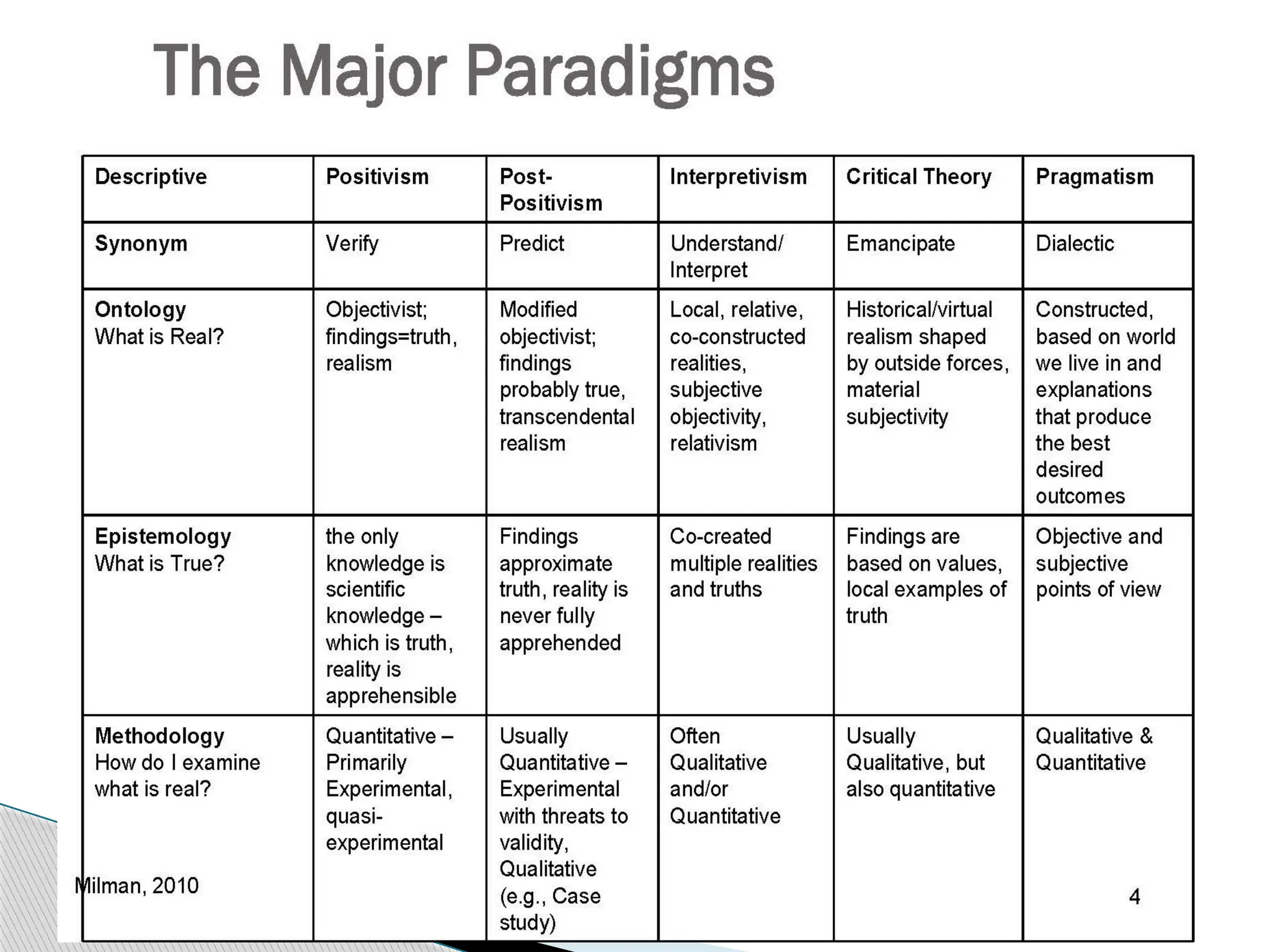
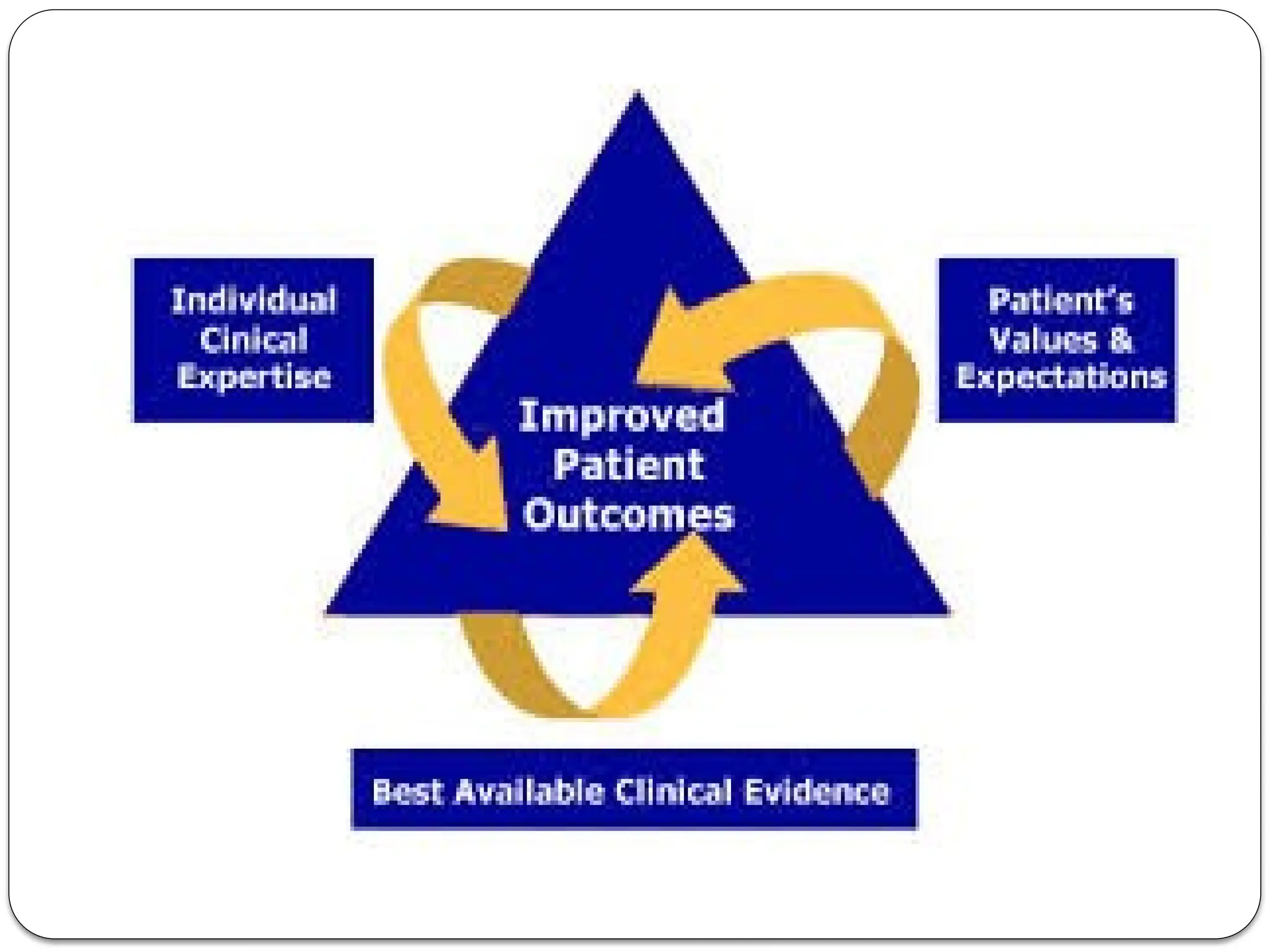
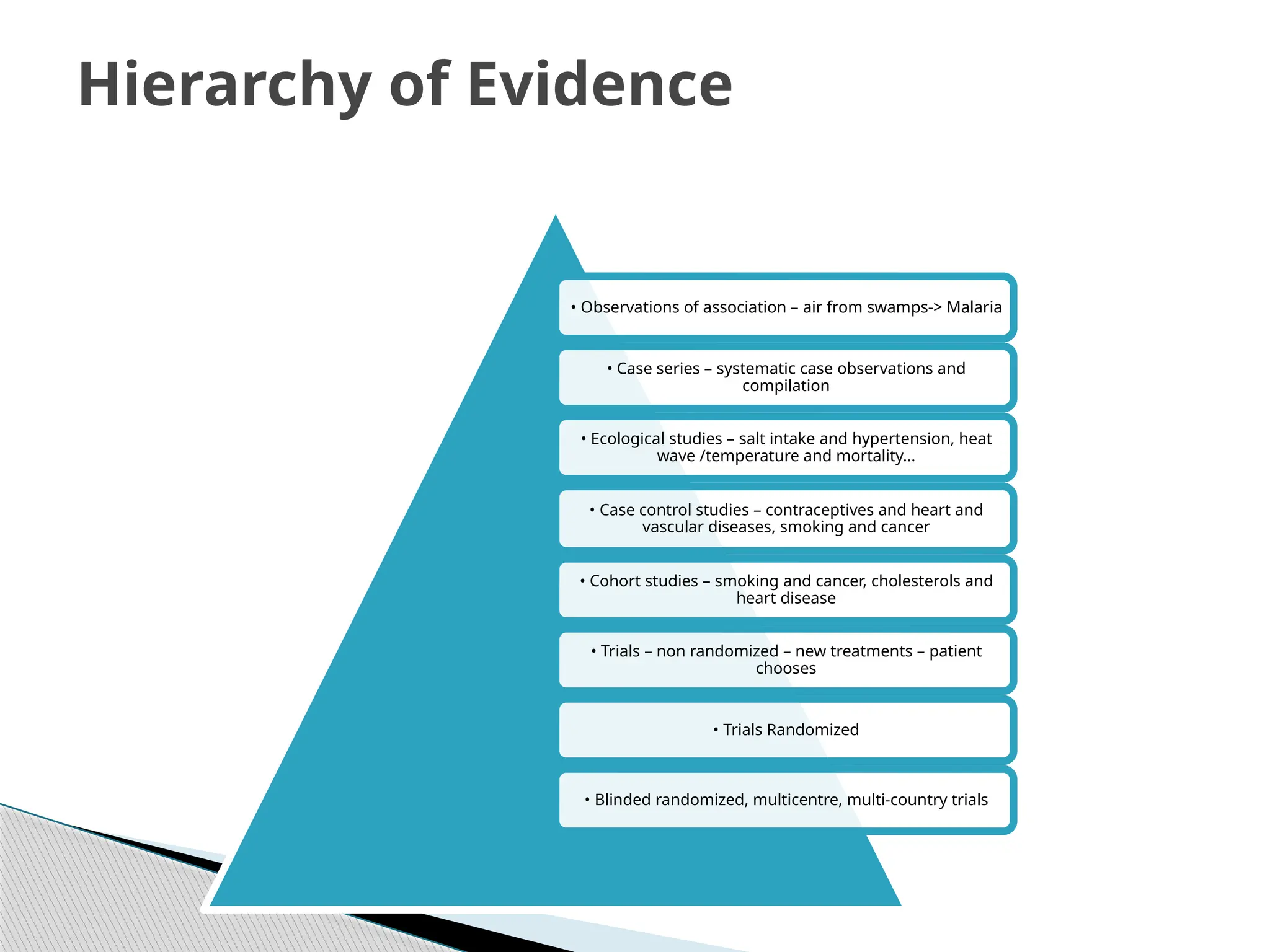
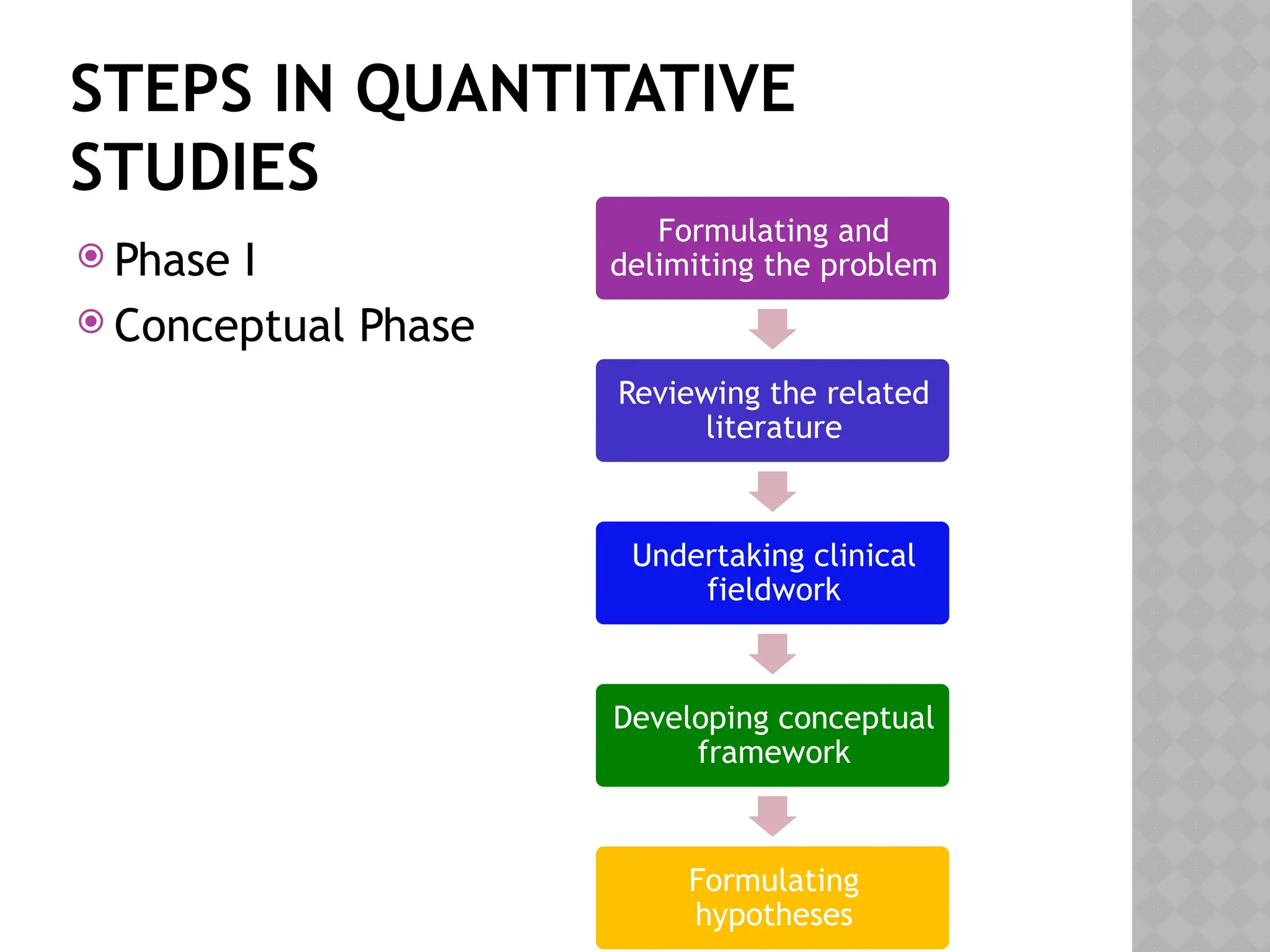
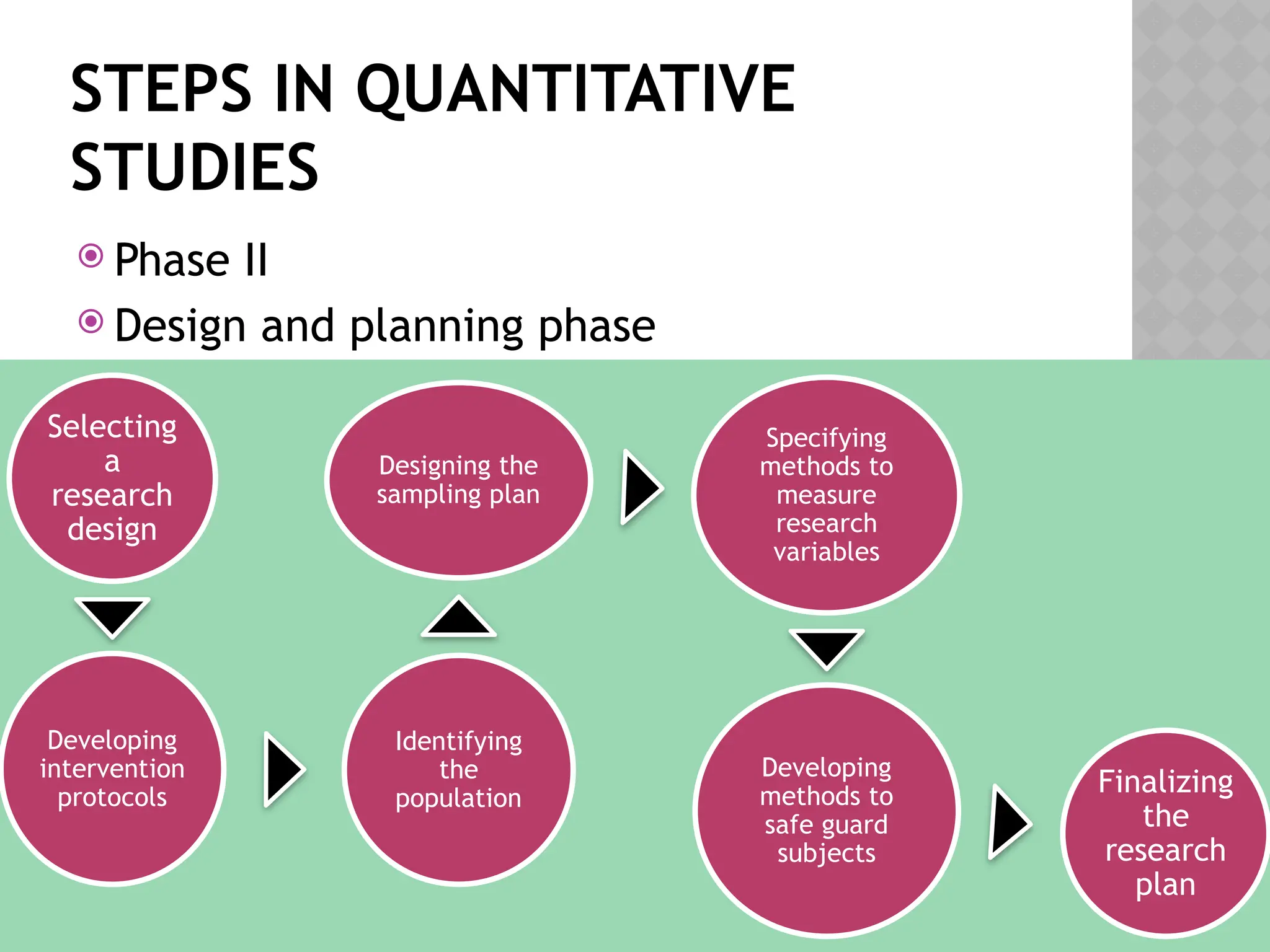
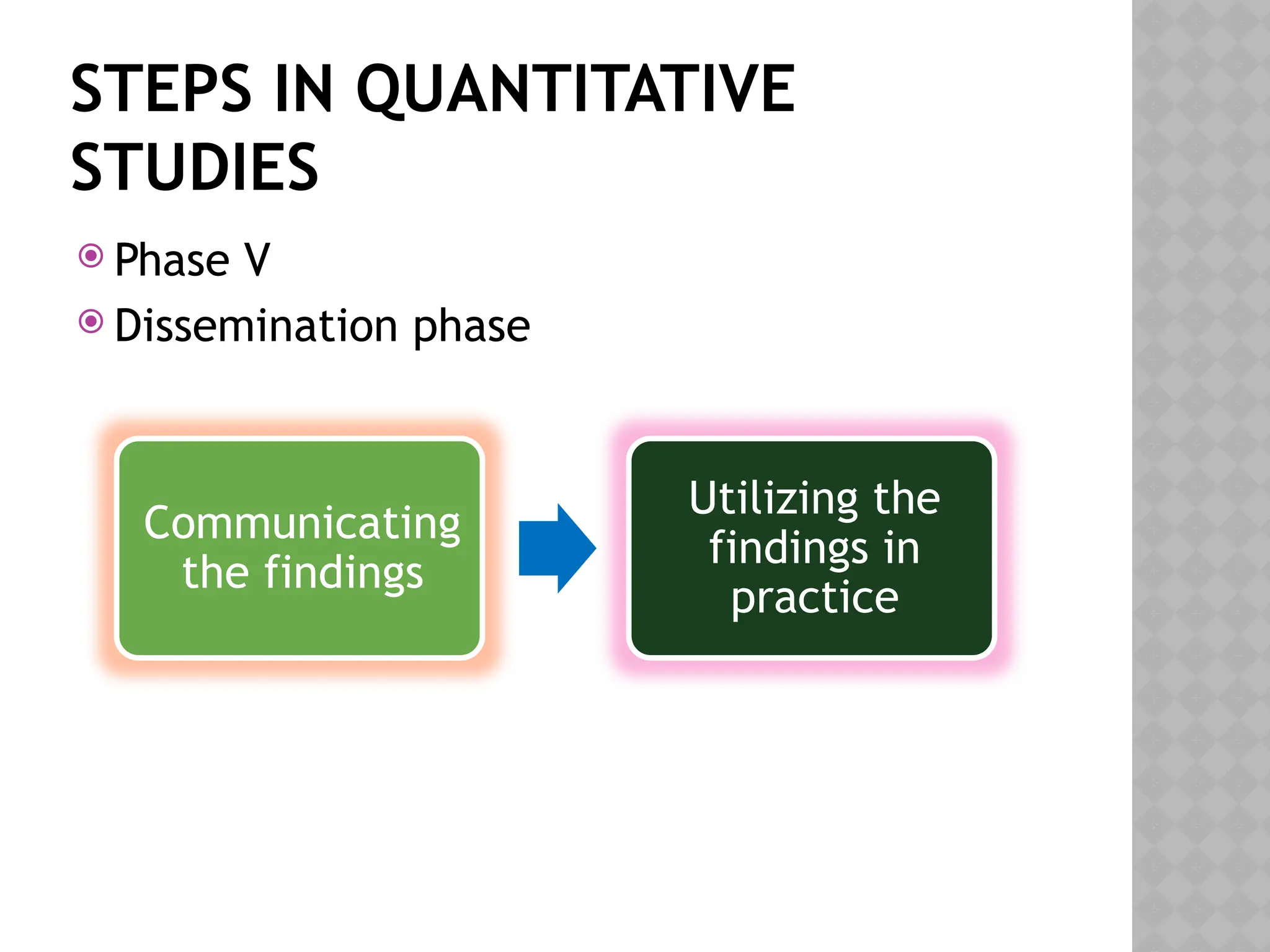
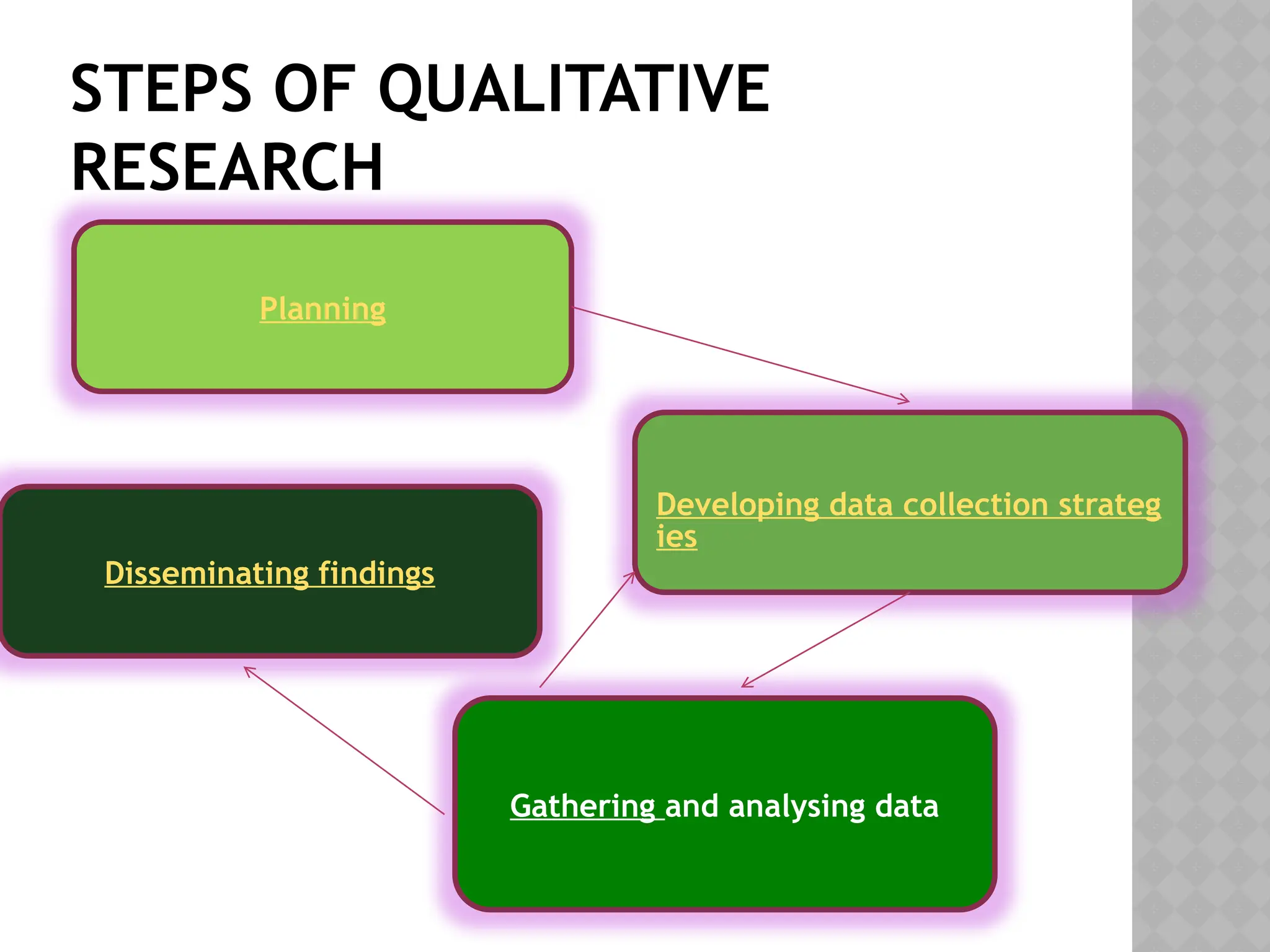
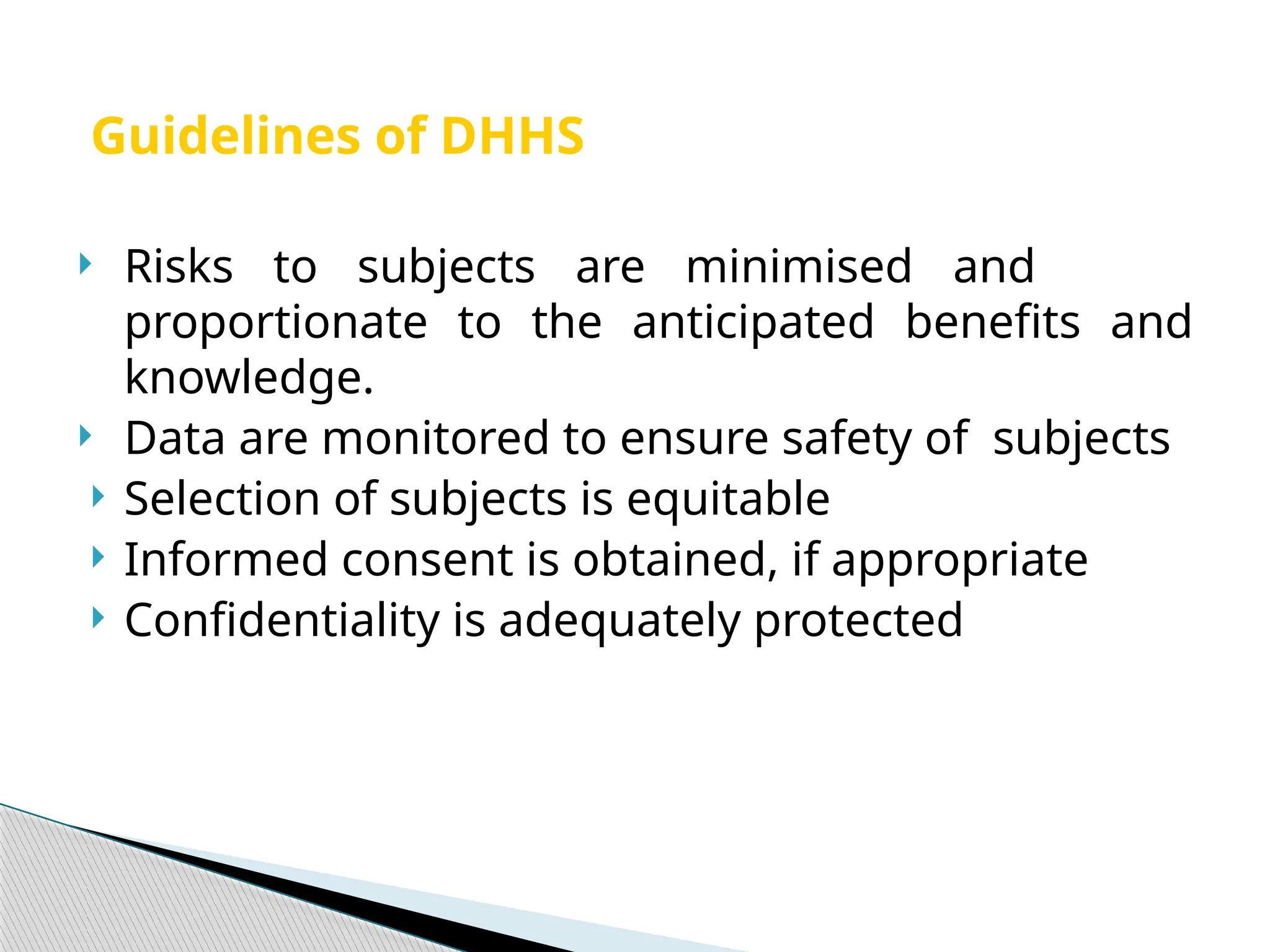
The document provides an extensive review of nursing research, covering its definition, significance, historical evolution, types, and applications in practice. It outlines various research methodologies, challenges faced in nursing research, the importance of evidence-based practice, and future trends within the field. It emphasizes ethical considerations and the role of scientific inquiry in solving health-related issues and developing nursing practices.