Embed presentation
Downloaded 24 times


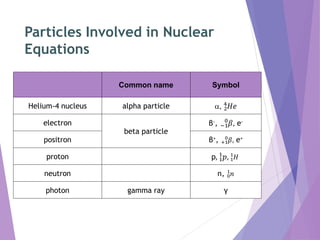
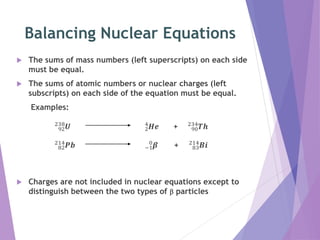
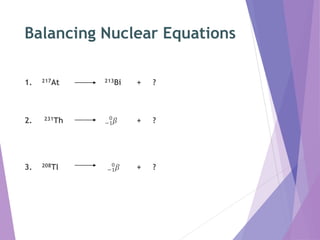
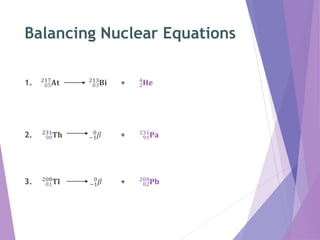
Nuclear reactions involve changes to the nuclei of atoms through the emission or absorption of particles such as alpha particles, beta particles, protons and neutrons. This results in large changes in mass and huge amounts of energy. Nuclear equations must balance the mass numbers on each side and the nuclear charges to show the nuclear reaction. Examples are given of balancing nuclear equations involving alpha and beta particle emission.





