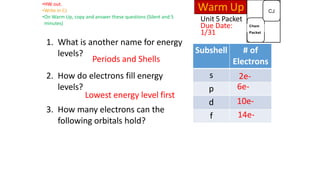
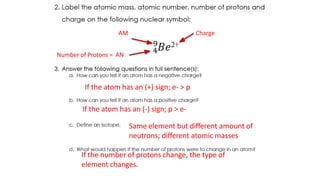
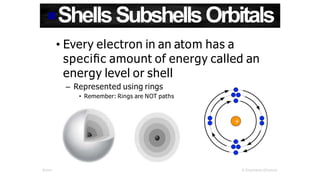
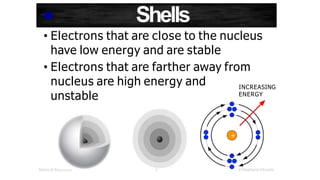
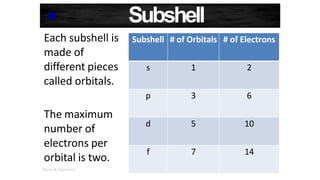
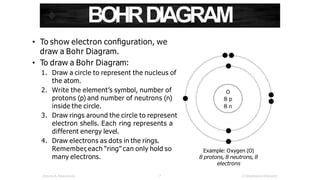
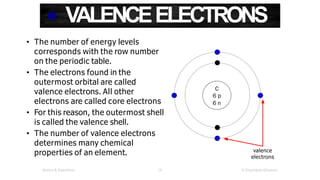
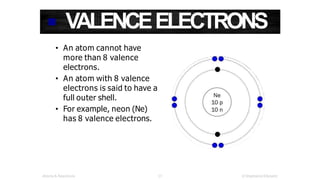
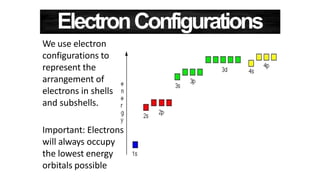
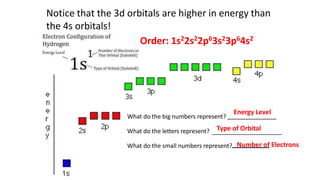
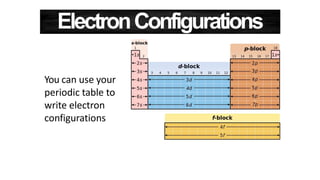
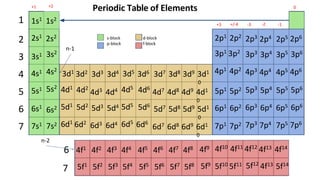
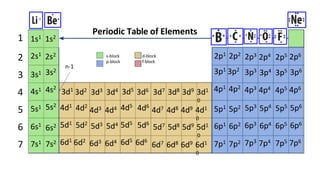
The document discusses electron shells and orbitals. It explains that electrons occupy specific energy levels called shells, with each shell divided into subshells. The subshells are labeled s, p, d, and f, and can hold a maximum number of electrons. Electron configurations are used to represent the arrangement of electrons in shells and subshells, following the rule that electrons fill the lowest energy orbitals first. Valence electrons are those in the outermost shell and determine an element's chemical properties. Bohr diagrams use circles and dots to visually depict electron arrangements of atoms.














![Examples
** Follow your chart – this is important when writing
out configurations for elements past Ca **
Sodium (Na):
Longhand:
Noble Gas: [Ne]3s1
Phosphorus (P):
1s22s22p63s1
Change this to
Phosphorus (P)
Ne = 1s22s22p6
Ne = 1s22s22p6
Longhand: 1s22s22p63s2 3p3
Noble Gas:[Ne] 3s23p3](https://image.slidesharecdn.com/noteselectronsandelectronconfig-231021004818-56eb7a71/85/Notes-Electrons-and-Electron-Config-pptx-26-320.jpg)