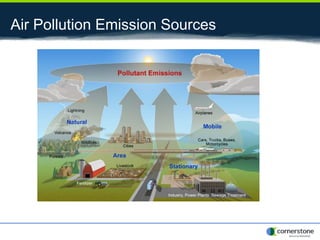
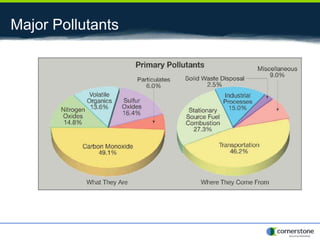
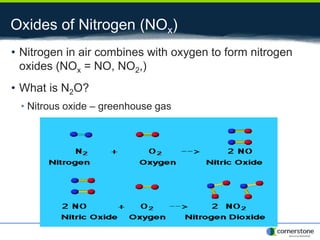
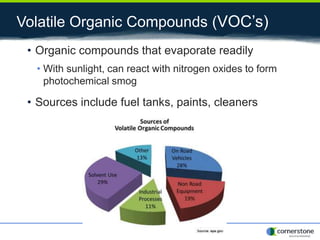
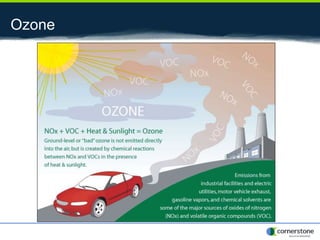
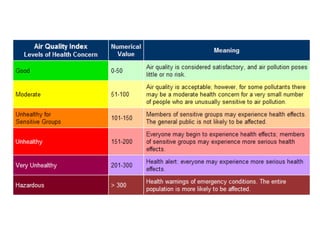
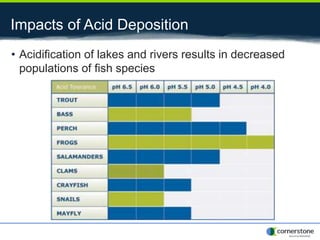
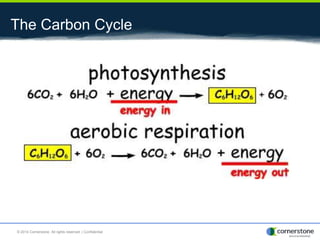
The document provides an overview of air pollution, detailing various pollutants such as nitrogen oxides, volatile organic compounds, and greenhouse gases, along with their health and environmental effects. It discusses regulatory frameworks established by the Clean Air Act and the National Atmospheric Deposition Program to reduce emissions and improve air quality. Additionally, it highlights the significance of understanding the carbon cycle and greenhouse gas reporting requirements for facilities to mitigate climate change impacts.