1. The document discusses mucosal vaccine delivery systems, including their advantages over injectable vaccines. It describes various mucosal delivery methods like emulsion-type, liposome-based, polymeric nanoparticles, virosomes, and melt-in-mouth strips.
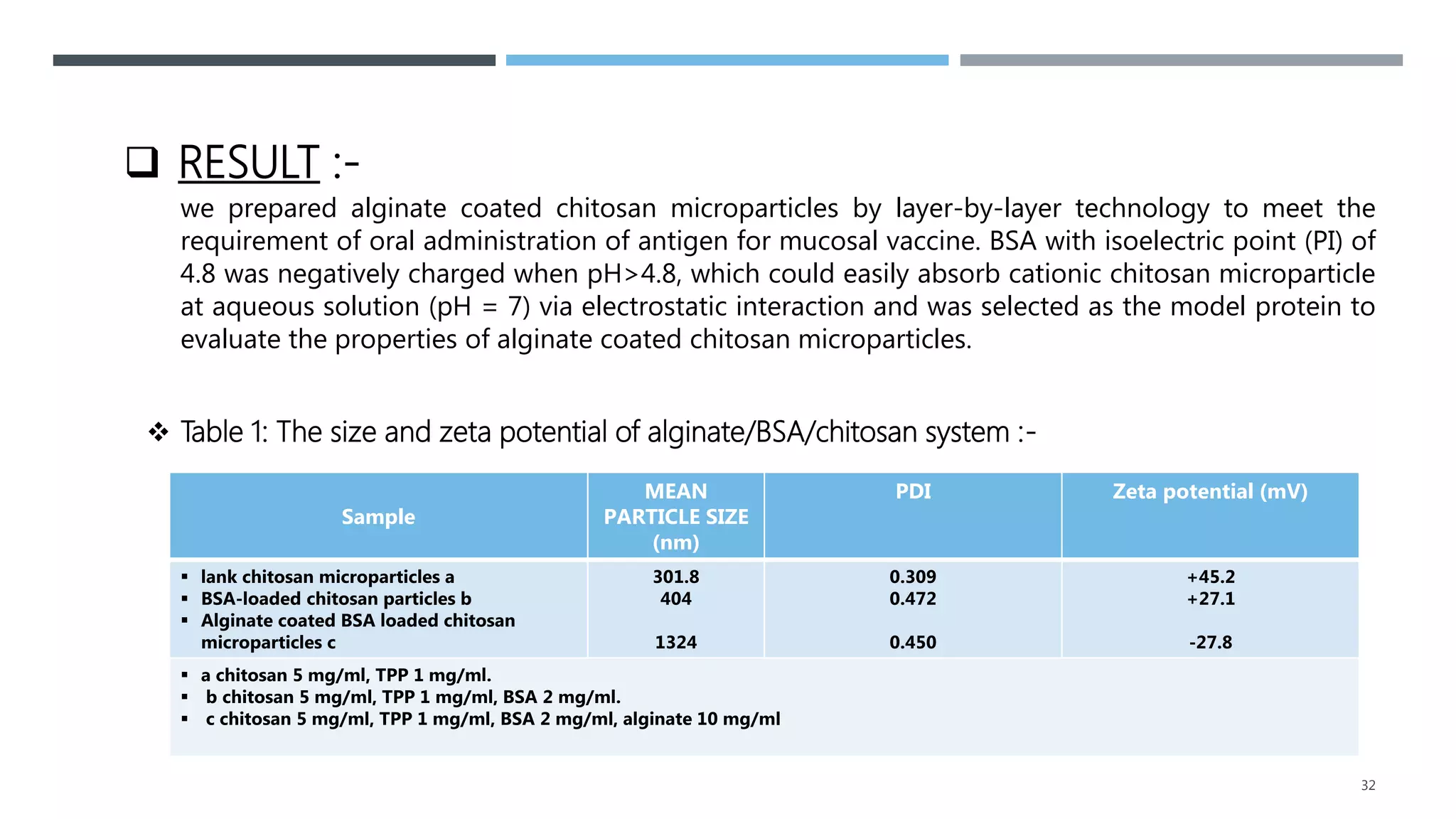
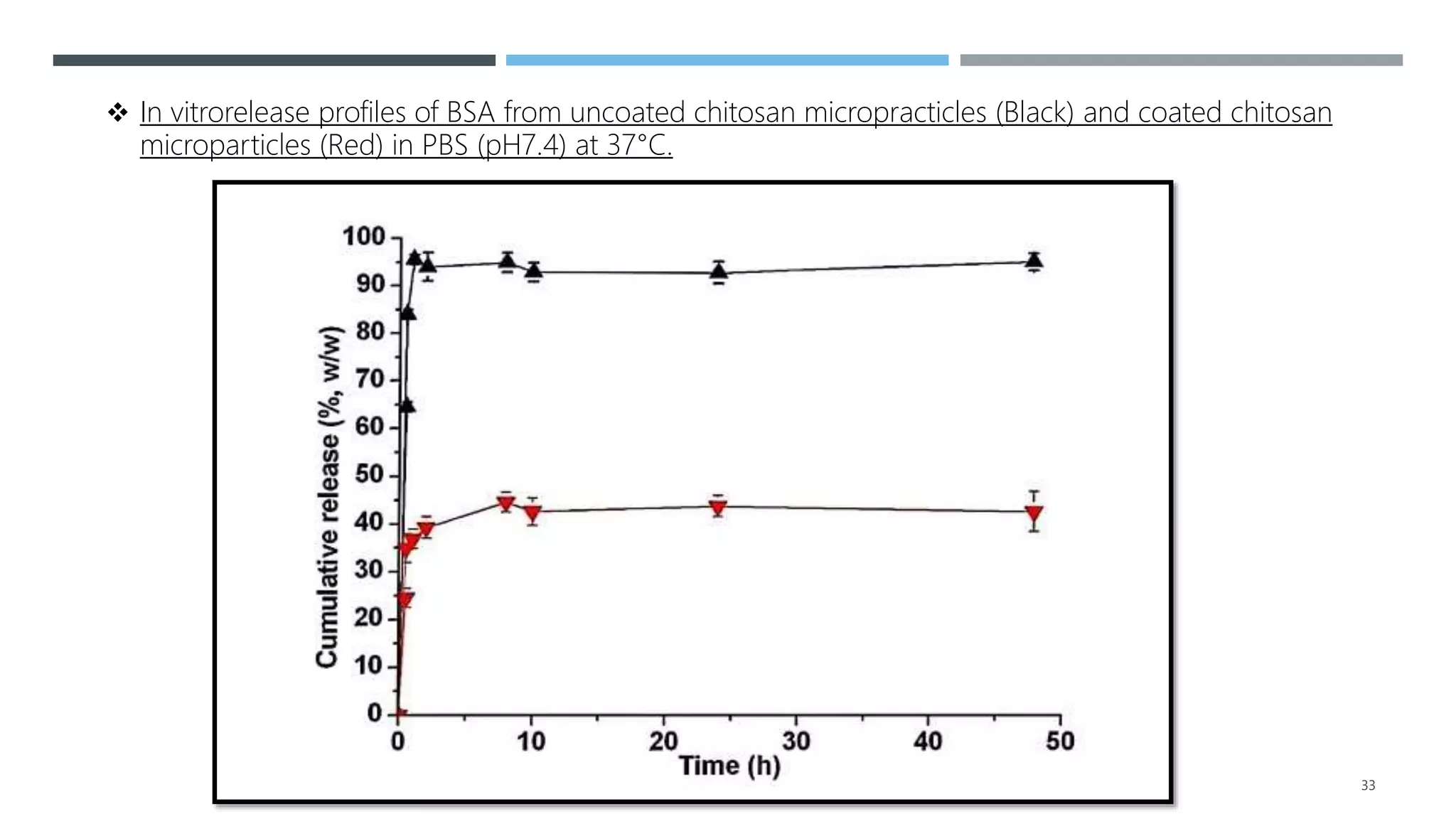
2. It summarizes a case study on the preparation of alginate-coated chitosan microparticles for vaccine delivery and their ability to modulate antigen release and protect from degradation.
3. Overall, the document outlines the promise of mucosal vaccines for improved compliance and induction of mucosal immunity, but also challenges like rapid clearance that must be addressed through innovative delivery systems and adjuvants.

![INTRODUCTION
Vaccination against infectious diseases has proven to be an asset
in preventing diseases and has contributed significantly to an
increase in life expectancy. [1]
It is believed that the first productive interaction among the most
infectious agents and that host is with mucosal surfaces, especially
the agents and the host is with mucosal surfaces, especially the
nasal, oropharyngeal, respiratory, genitourinary and
gastrointestinal mucosa. [2]
2](https://image.slidesharecdn.com/mucosaldds-210601064225/75/Mucosal-dds-2-2048.jpg)
![ MUCOSAL DELIVERY OF VACCINES
Mucosal surfaces area is major portal of entry for many human
pathogens that are the cause of infectious diseases
worldwide.[3]
Immunization by mucosal routes may be more effective at
inducing protective immunity against mucosal pathogens at
their sites of entry.[4]
Efforts have focused on efficient delivery of vaccines antigens to
mucosal sites that facilitate uptake by local antigen-presenting
cells to generate protective mucosal immune responses. [5] 4](https://image.slidesharecdn.com/mucosaldds-210601064225/75/Mucosal-dds-4-2048.jpg)

![MUCOSAL TYPE
The adult human mucosa lines the surfaces of the digestive,
respiratory and genitourinary tracts, covering an immune
surface area that is nearly 200 times greater than that of the
skin. It is estimated that 70% of the infectious agents enter
the host by mucosal routes.
Mucosal surfaces are typically categorized as type-I and
type-II mucosa.
Type-I mucosa includes surface area of lungs and gut.[6]
6](https://image.slidesharecdn.com/mucosaldds-210601064225/75/Mucosal-dds-6-2048.jpg)
![ Type-II mucosa include surface area of mouth, esophagus and
cornea.
The female genital tract has both type-I and type-II mucosa.
Most mucosal sites have organized lymphoid follicles, such as
NALT, and GALT, which have assembly of scattered antigen-
reactive cells of immune system, such as B cells, T cells, and
professional antigen presenting cells such as dendritic cells(APCs).
It is widely accepted that mucosal vaccination can induce immune
responses at both systemic and mucosal sites and, prevent the
invasion and colonization of pathogens at mucosal surfaces.[7] 7
CONTINUE……](https://image.slidesharecdn.com/mucosaldds-210601064225/75/Mucosal-dds-7-2048.jpg)

![ CONTRIBUTION OF POLYMERS IN
MUCOSAL VDS.
The concept of polymeric carrier system(s) offers advantage of
delivering drugs/antigens to a specific target site, where it has
to be released from the carrier.
Polymeric nanoparticles/microparticles can enhance the
immune response to mucosal administered antigens by several
means. [8]
9](https://image.slidesharecdn.com/mucosaldds-210601064225/75/Mucosal-dds-9-2048.jpg)

![EMULSION TYPE DELIVERY
Emulsions are heterogeneous liquid systems may be w/o or o/w.
Antigens are dissolved in a water phase and emulsified in the oil
in the presence of an appropriate emulsifier.
The controlled release characteristics of an emulsion are
determined by factors such as – Viscosity of oil phase – Oil to
water phase ratio – Emulsion droplet size. [9]
11](https://image.slidesharecdn.com/mucosaldds-210601064225/75/Mucosal-dds-11-2048.jpg)



















![5. Agger, E.M., Rosenkrands, I., Olsen, A.W., Hatch, G., Williams, A.,
Kritsch, C., Lingnau, K., von Gabain, A., Andersen, C.S., Korsholm,
K.S., and Andersen, P. 2006, Vaccine, 24, 5452.
6. Illum, l., Farraj, N.F., Fisher, A.N., Gill, L., Miglietta, M., and
Benedetti, L. 1994, J. Controlled Release, 29, 133.
7. Putney, S.D., and Burke, P.A. 1998, Nat. Biotechnol., 16 [published
erratum appears in Nat Biotechnol 1998 May;16(5):478], 153.
CONTINUE……
38](https://image.slidesharecdn.com/mucosaldds-210601064225/75/Mucosal-dds-38-2048.jpg)
