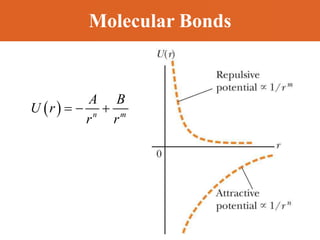
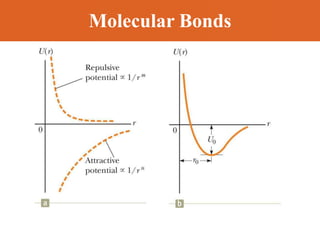
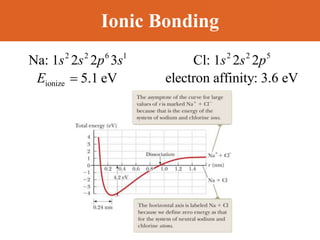
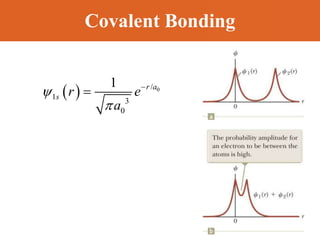
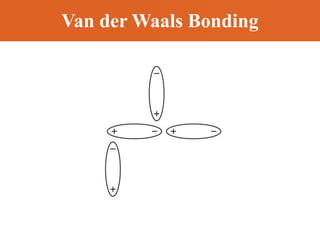
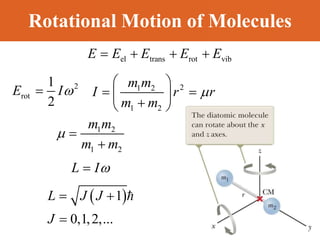
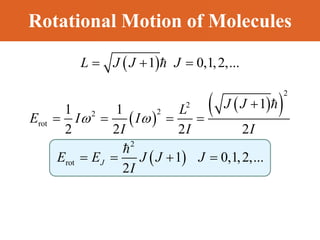
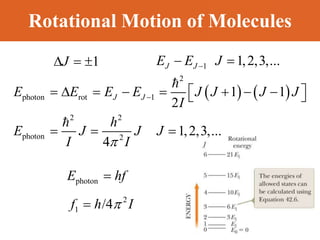
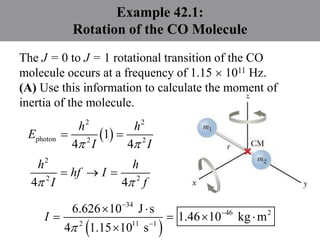
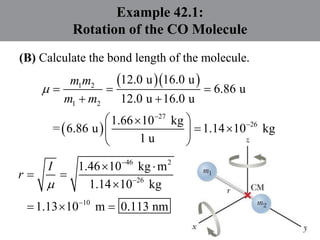
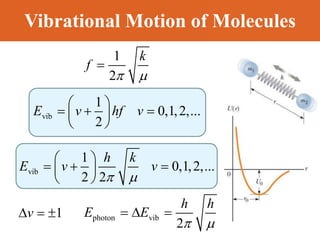
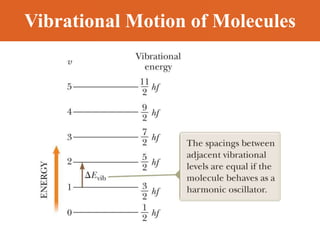
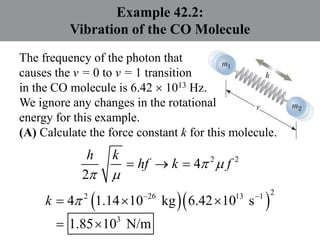
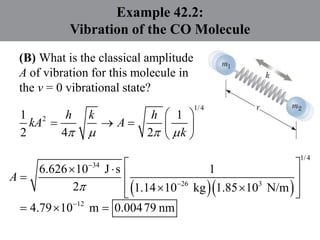
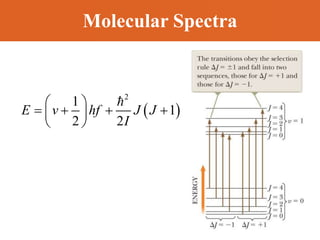
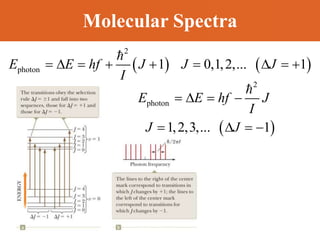
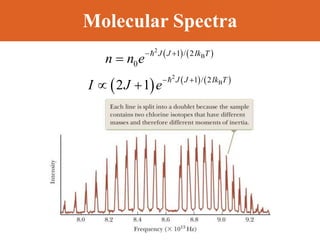
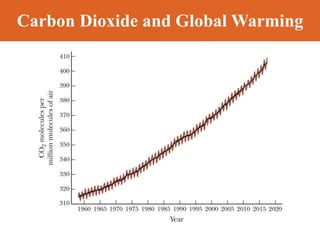
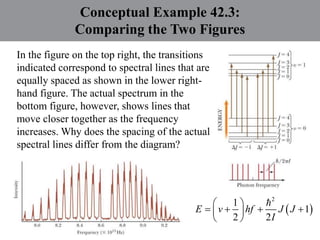
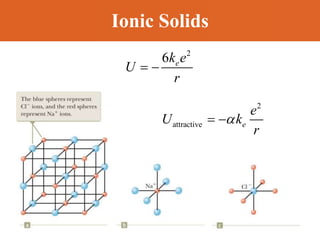
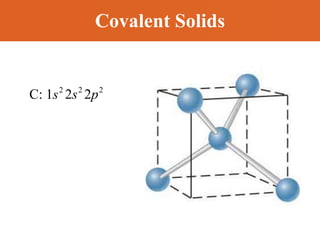
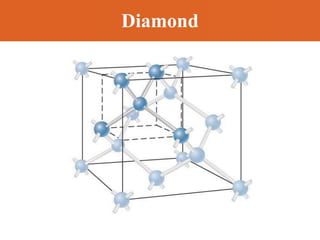
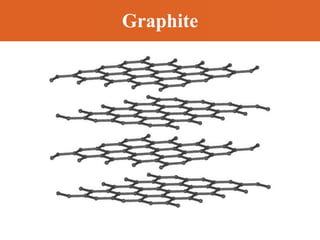
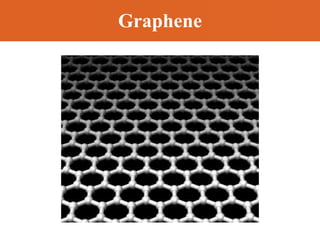
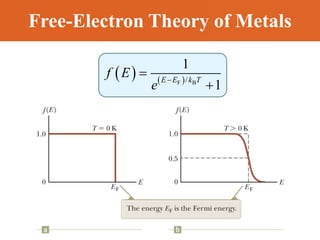
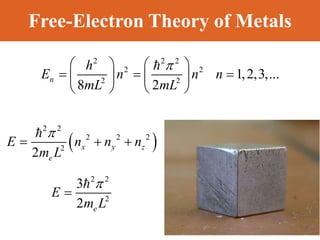
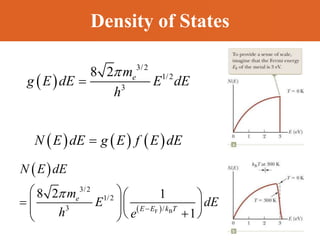
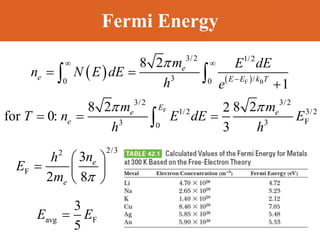
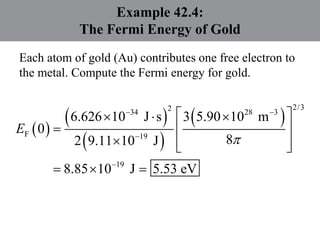
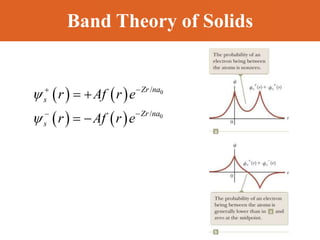
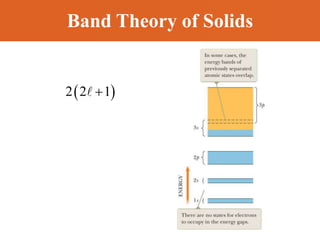
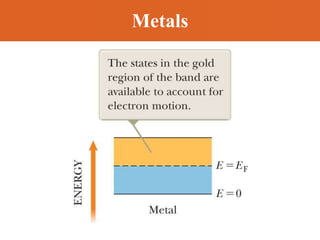
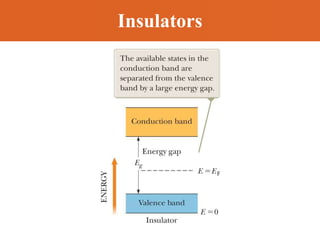
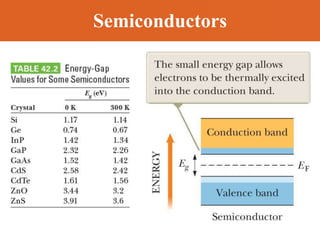
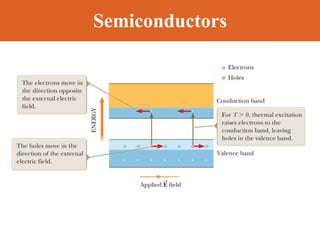
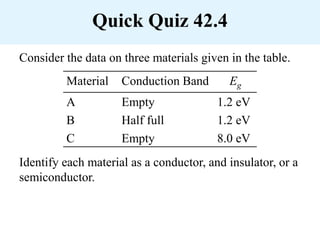
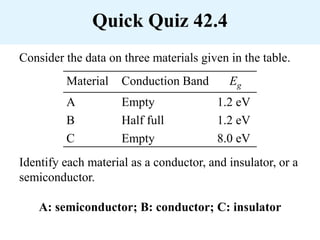
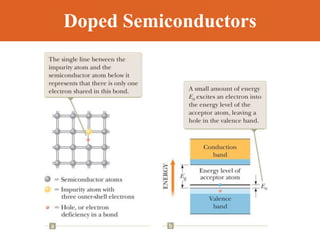
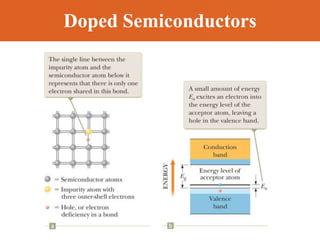
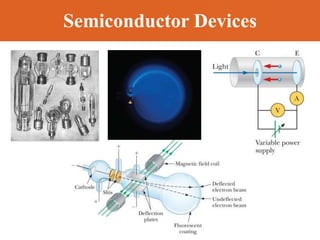
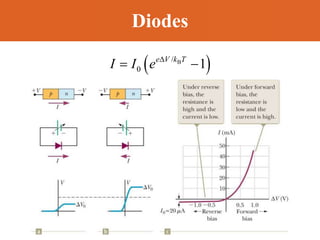
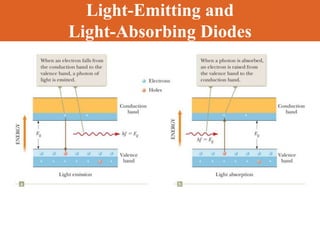
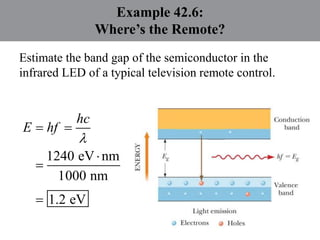
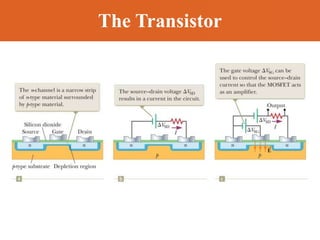
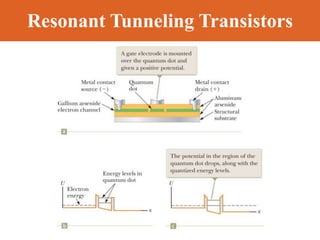
Chapter 42 discusses molecular bonds including ionic, covalent, and hydrogen bonding, with examples of each type provided. The chapter also covers molecular rotational and vibrational motions, including calculations related to energy transitions of molecules, along with quizzes for understanding. Additionally, it addresses the band theory of solids and materials classification, such as conductors, insulators, and semiconductors, with specific examples and calculations.