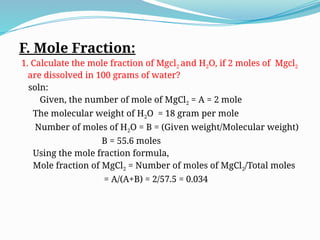
The document explains various concepts in chemistry, focusing on calculations of molarity, molality, normality, mass percentage, volume percentage, and mole fraction using specific examples. It provides step-by-step solutions to problems related to these concepts, demonstrating how to derive concentrations and percentages based on given quantities. Each section includes examples with clear formulas for performing the calculations.










![=[Volume of the solute/(Volume of the solute+Volume of the
solvent)]*100
= [35/(35+65)]*100
= (35/100)*100
= 35%
2. Calculate the volume of ethanol in 200ml solution of 20% v/v
aqueous solution of ethanol?
soln:
Given, Volume of aqueous solution = 200ml
Volume% = 20%
Using the formula of volume percentage,
20 = [Volume of ethanol(solute)/200]*100
Volume of ethanol = (20*200)/100 = 40ml](https://image.slidesharecdn.com/vaishnavi-250203154337-25a80f2c/85/Molarity-molality-and-normality-formula-and-their-calculation-11-320.jpg)