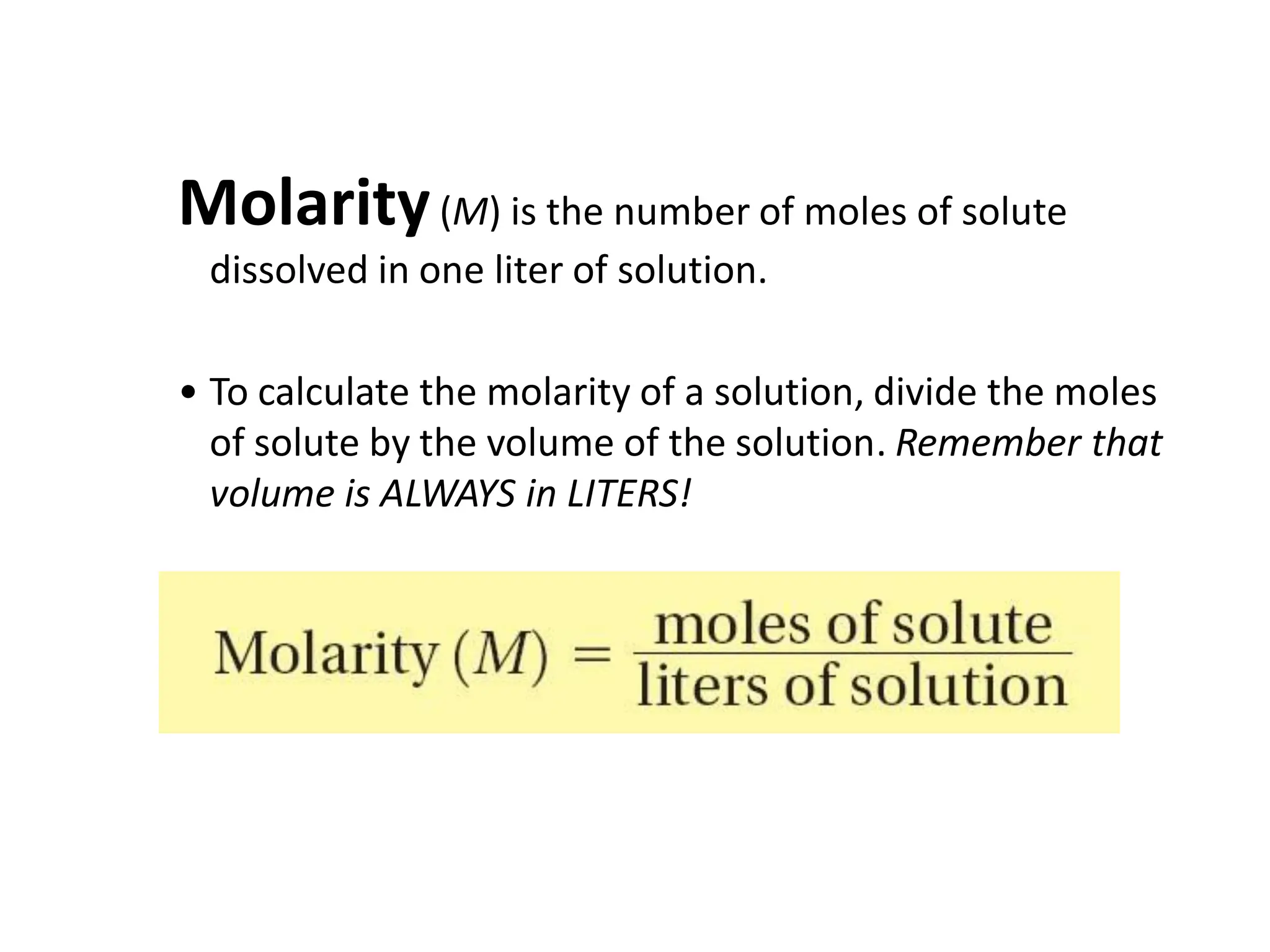
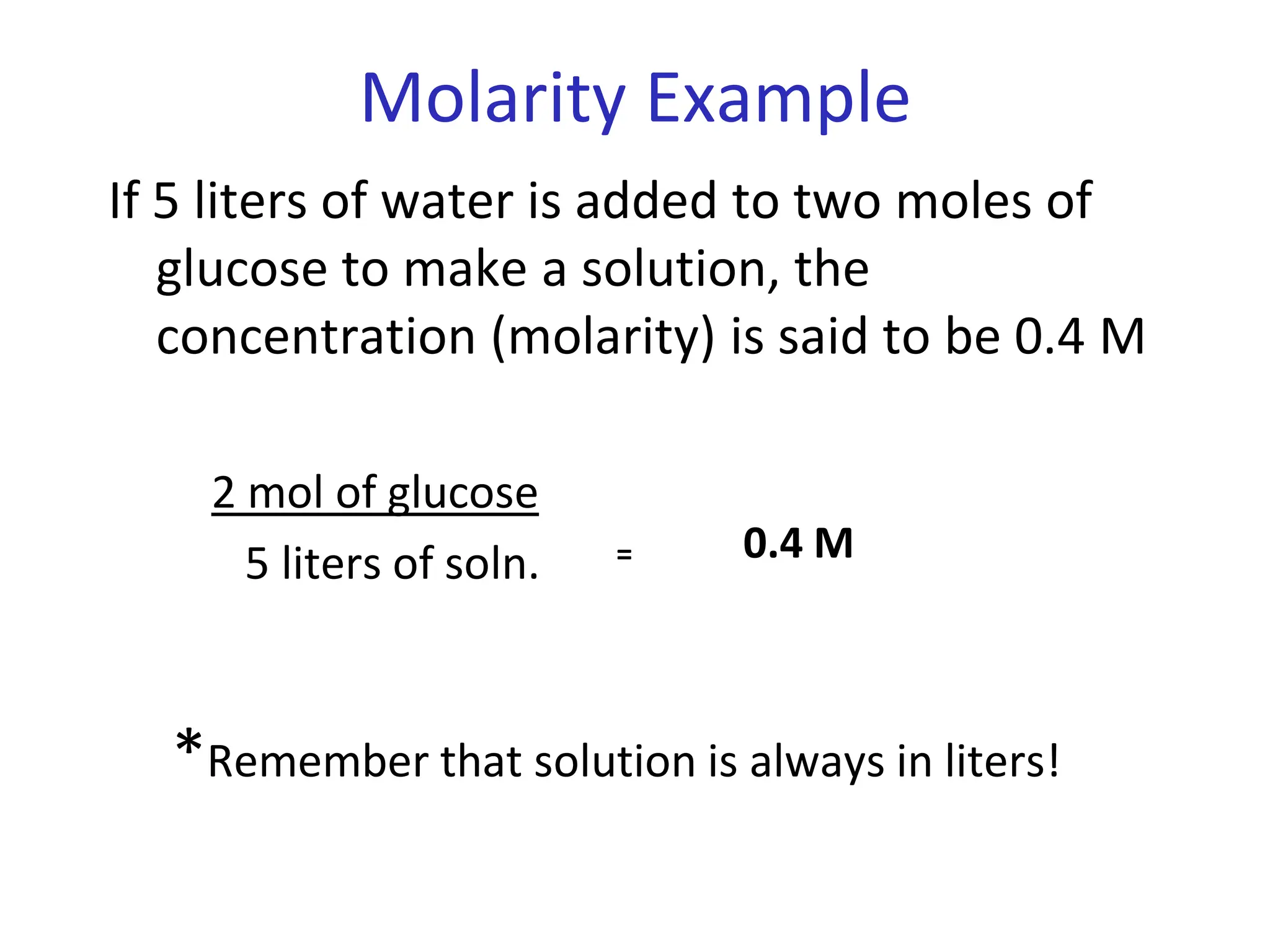
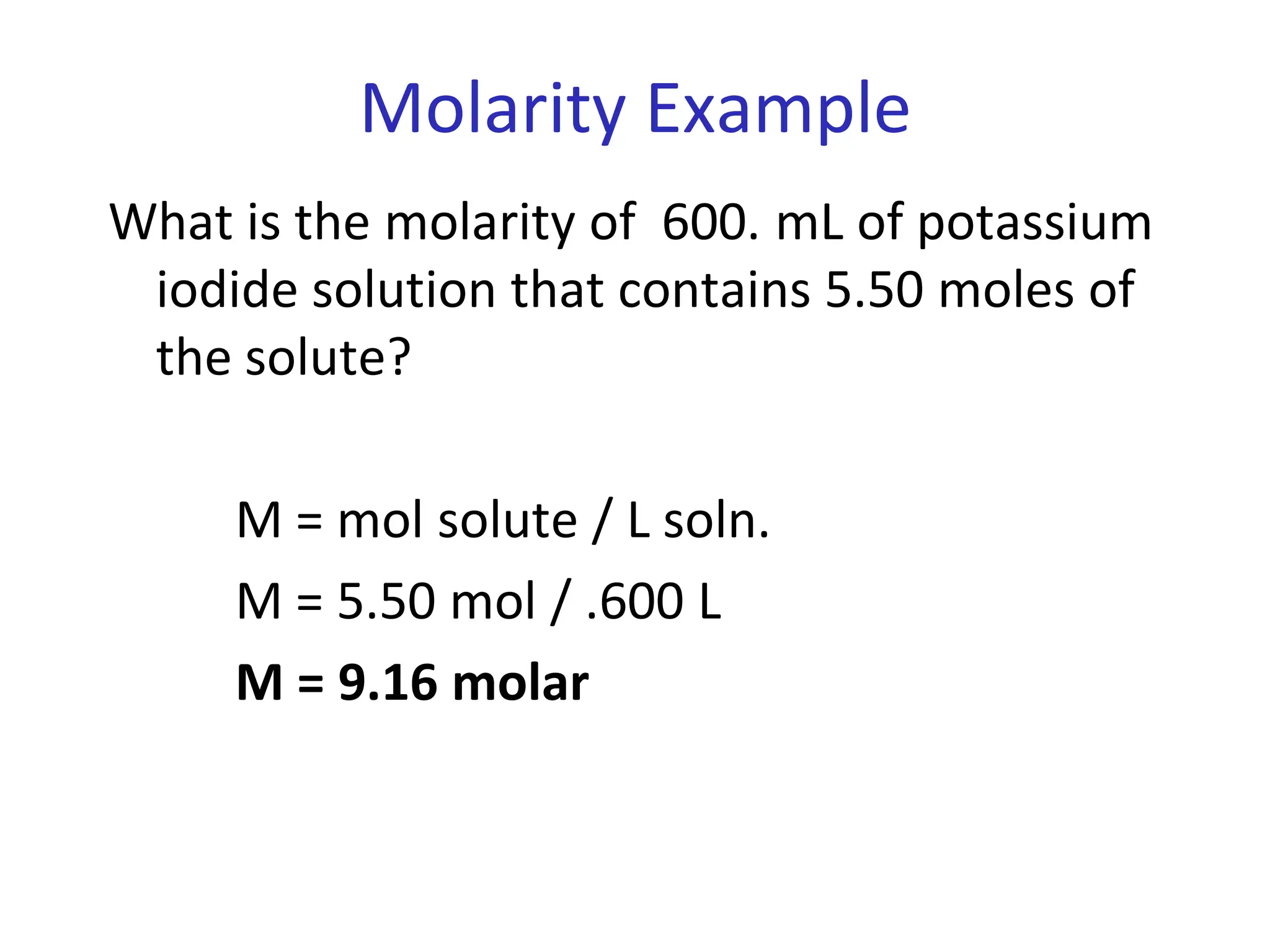
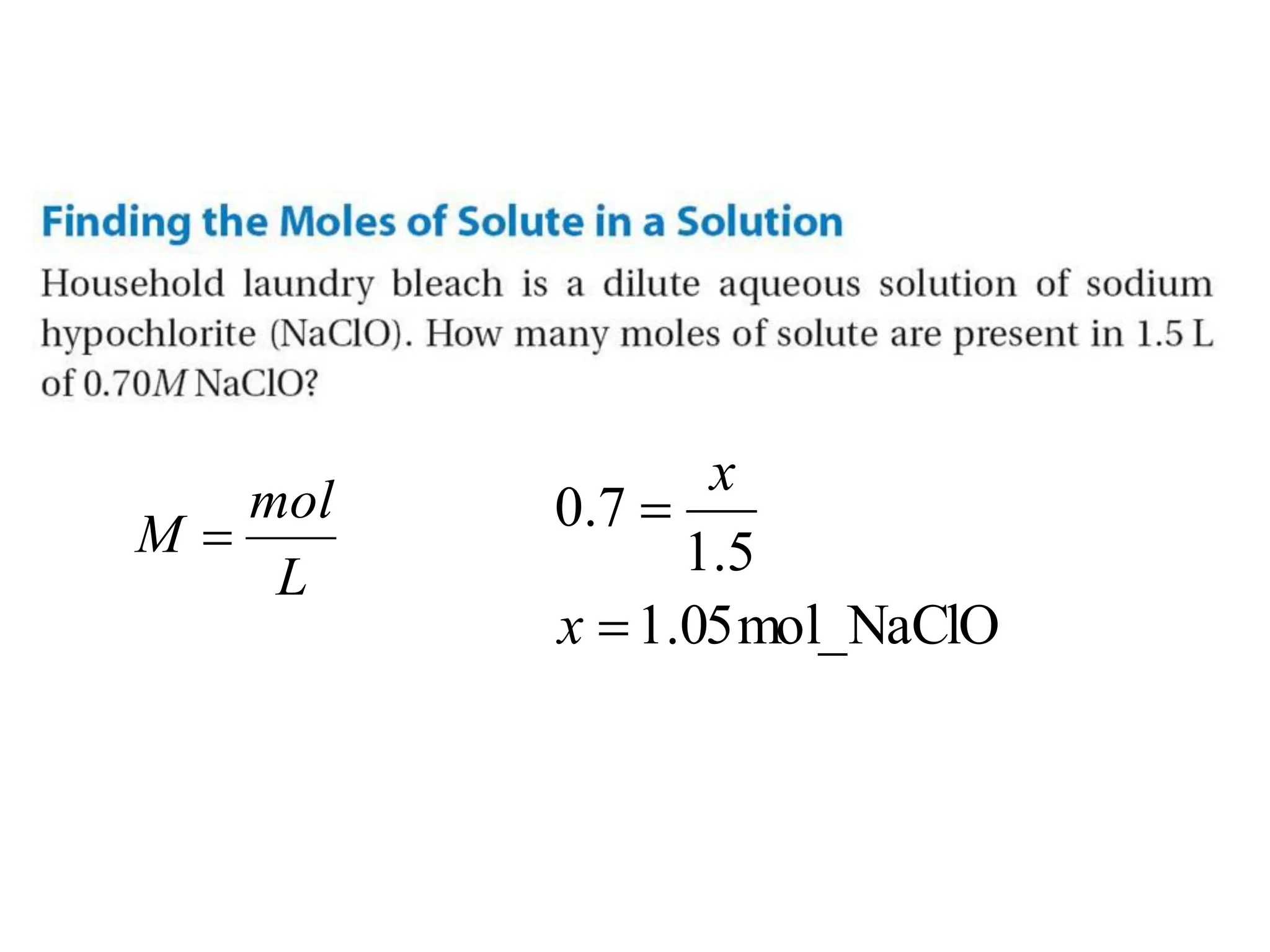
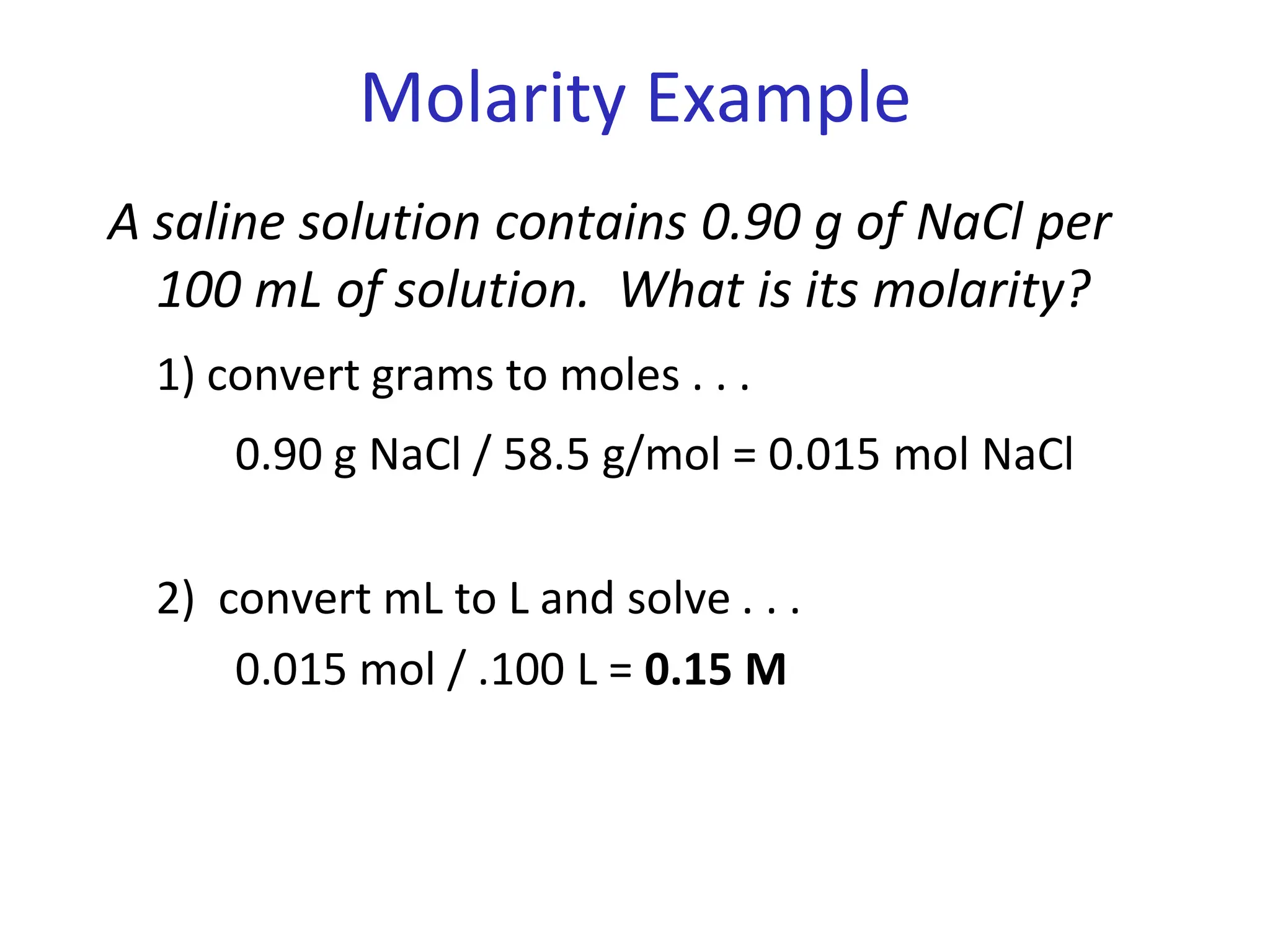
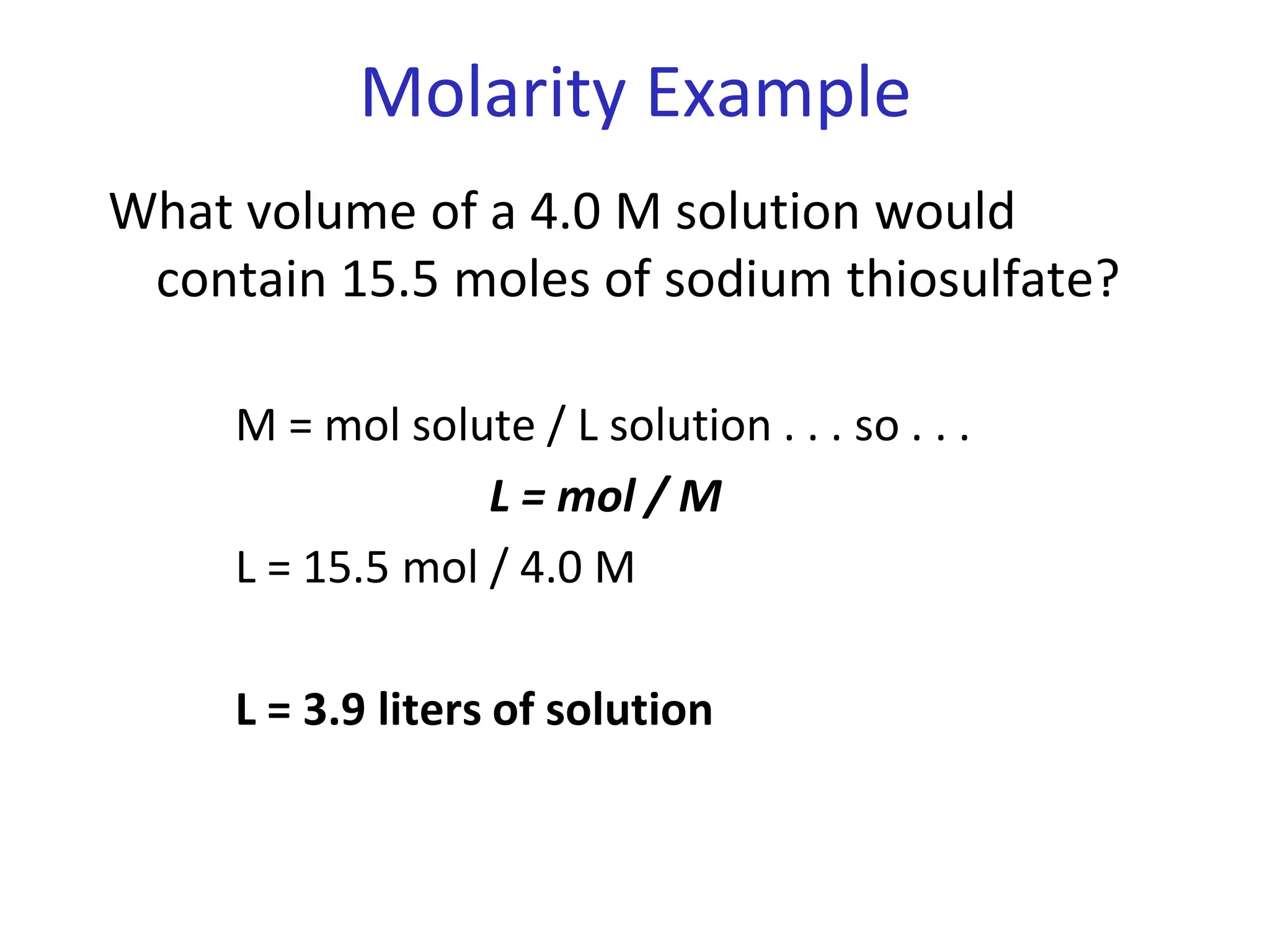
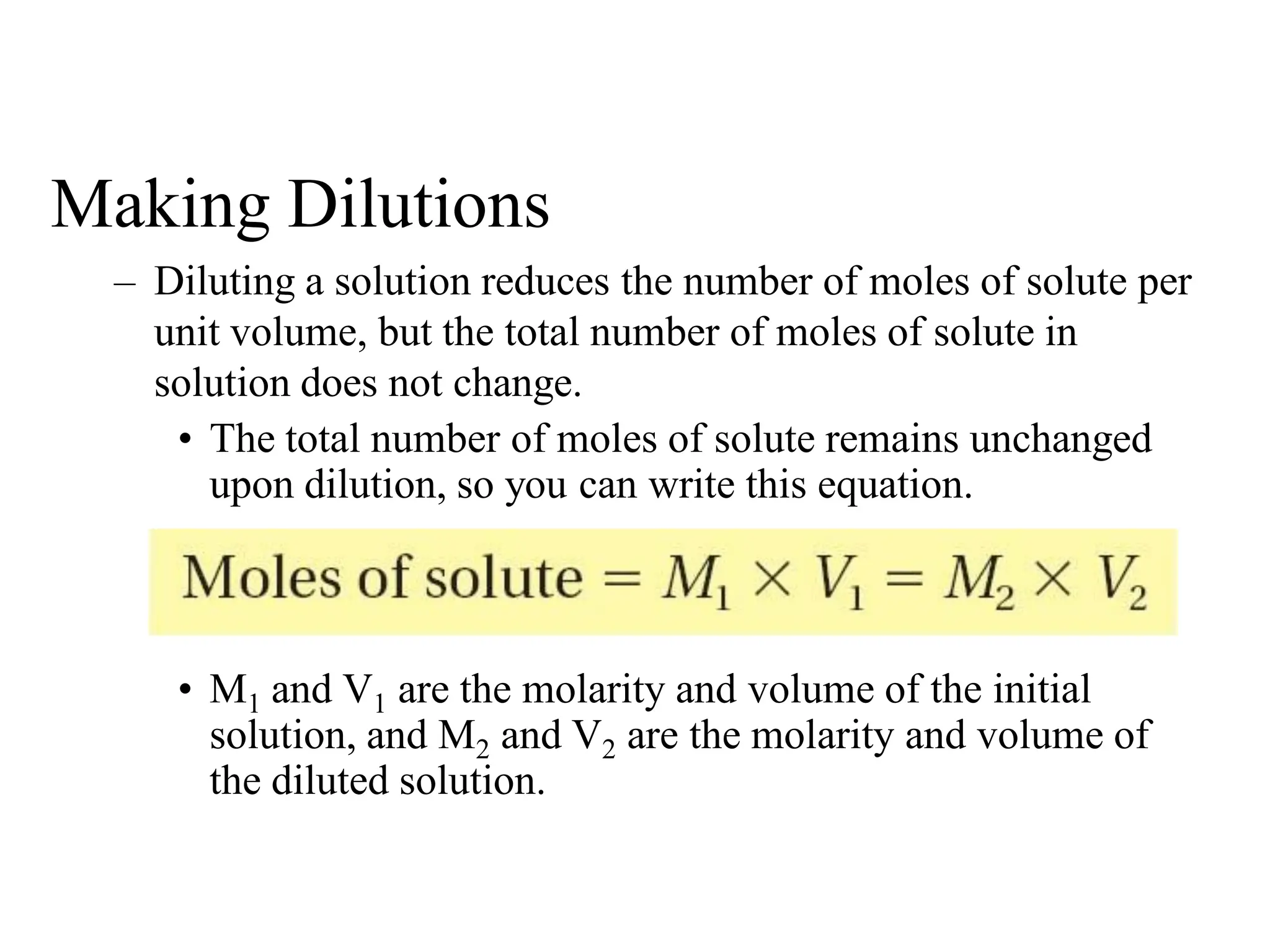
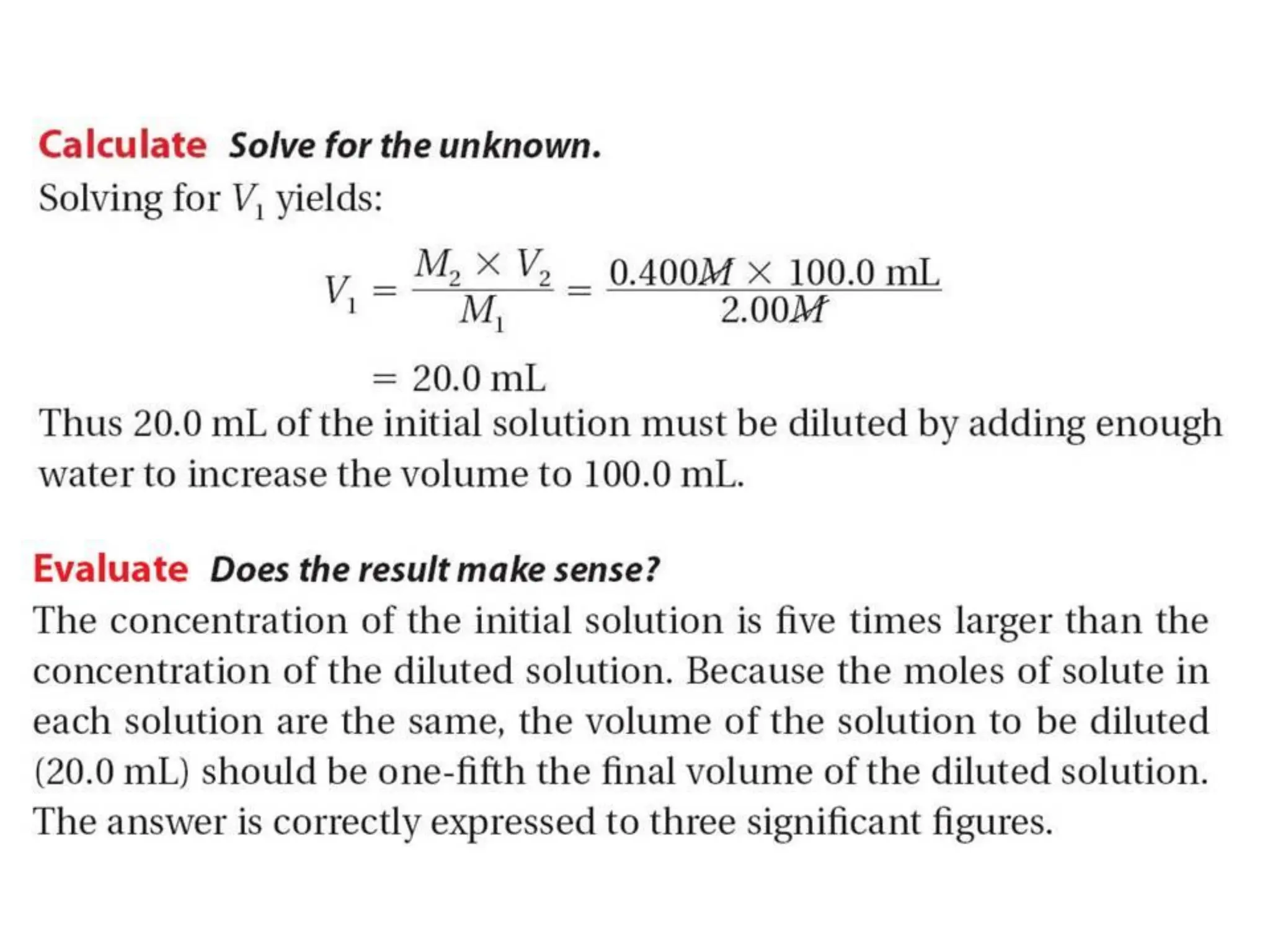
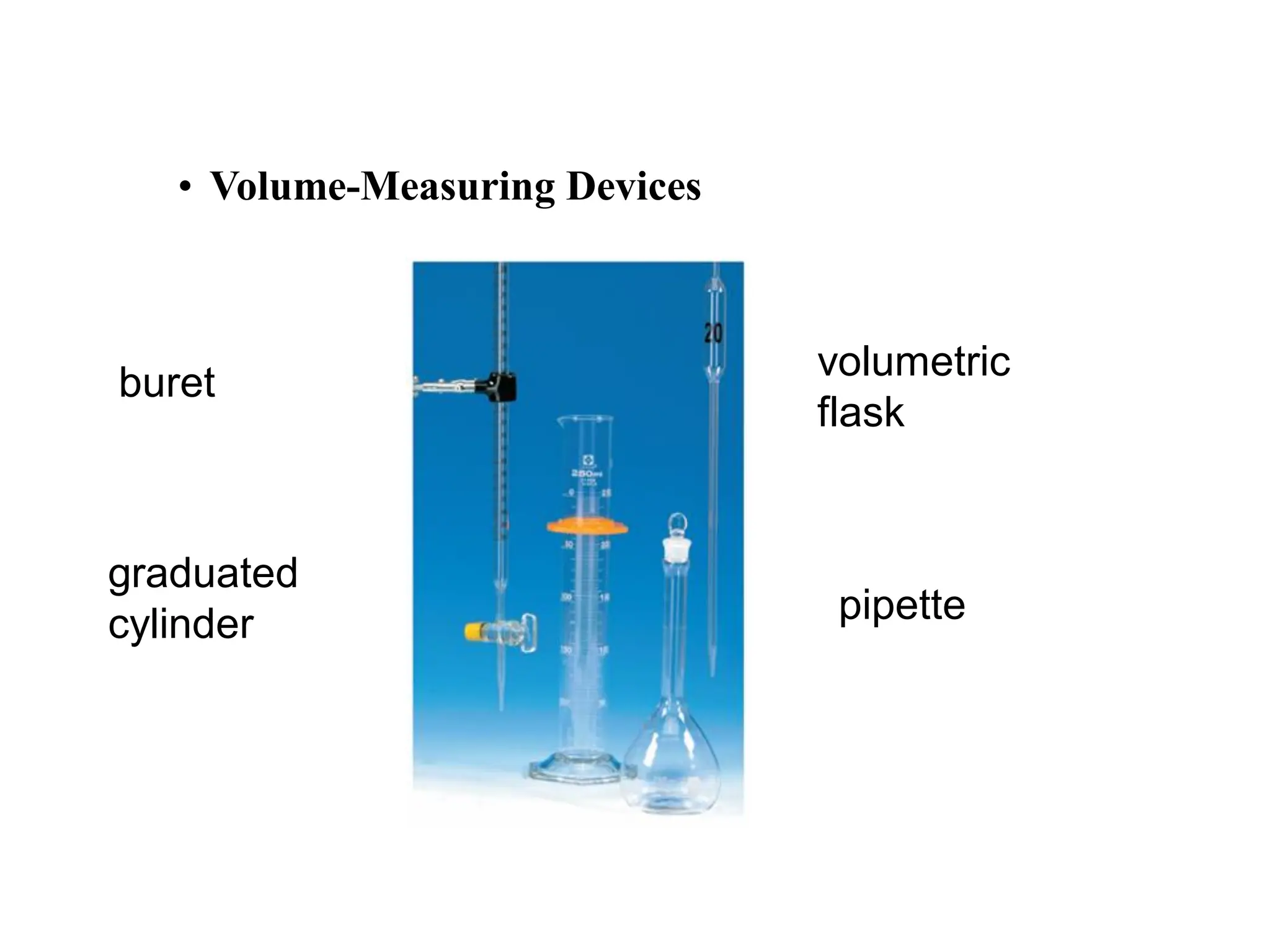
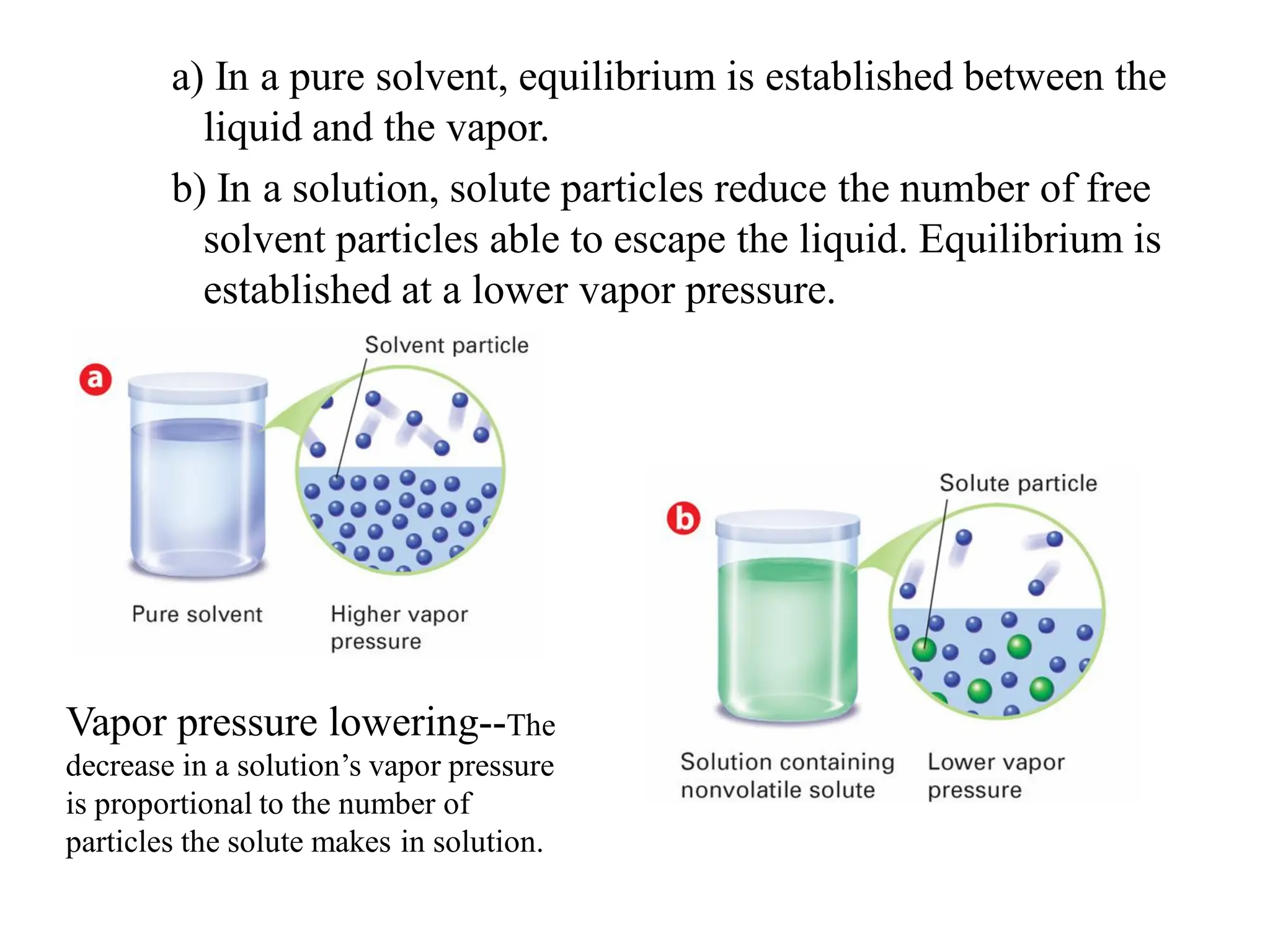
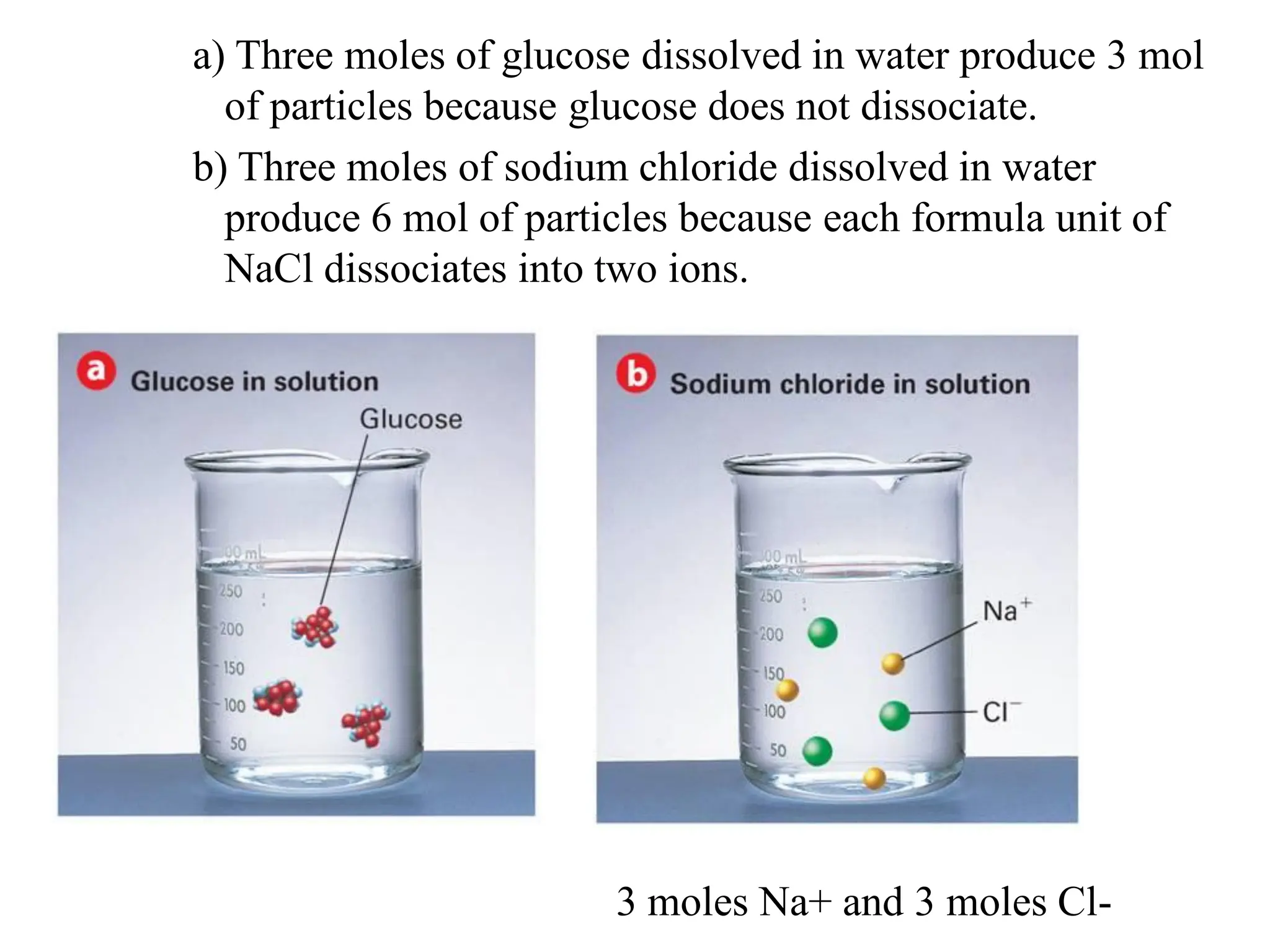
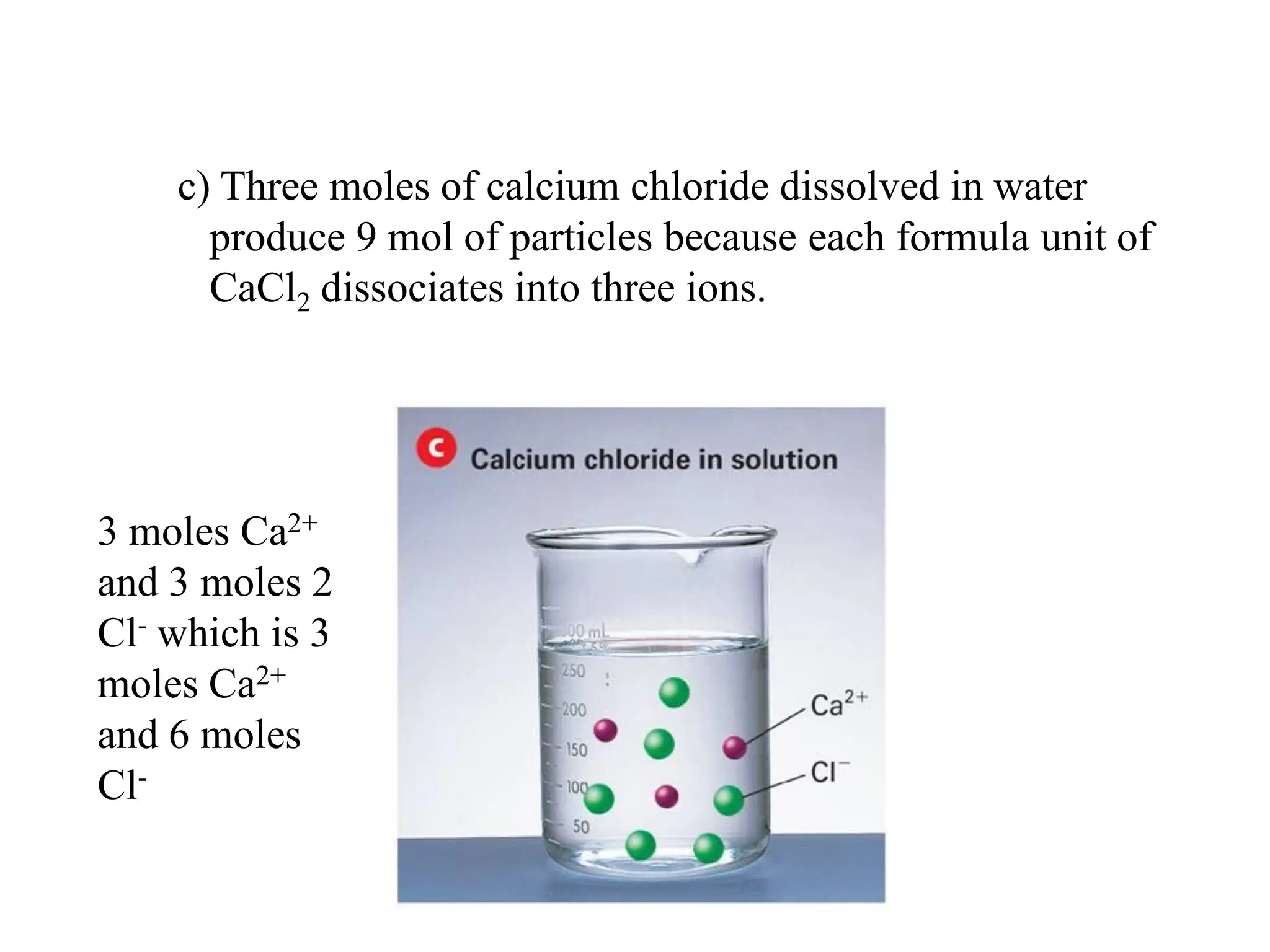
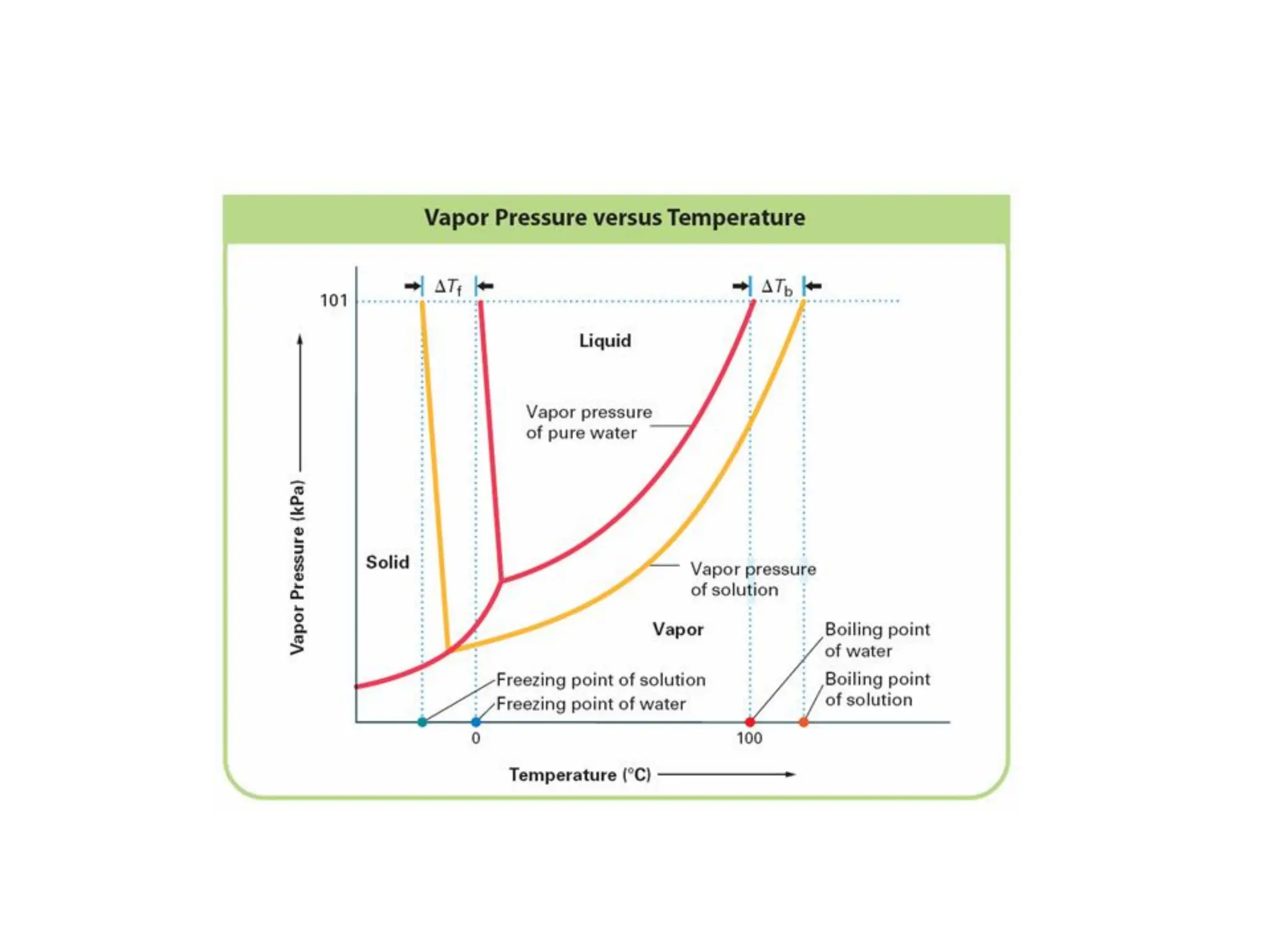
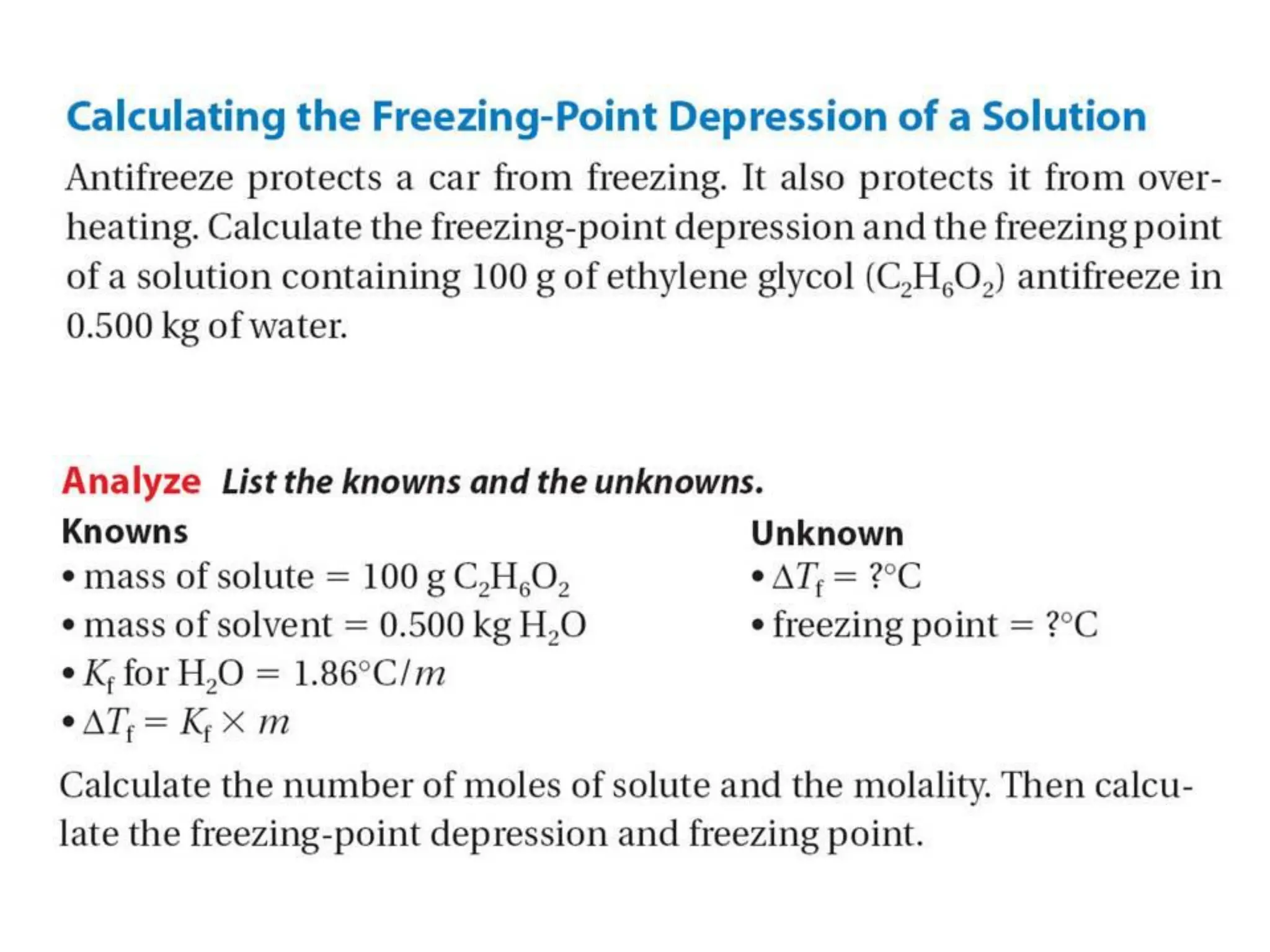
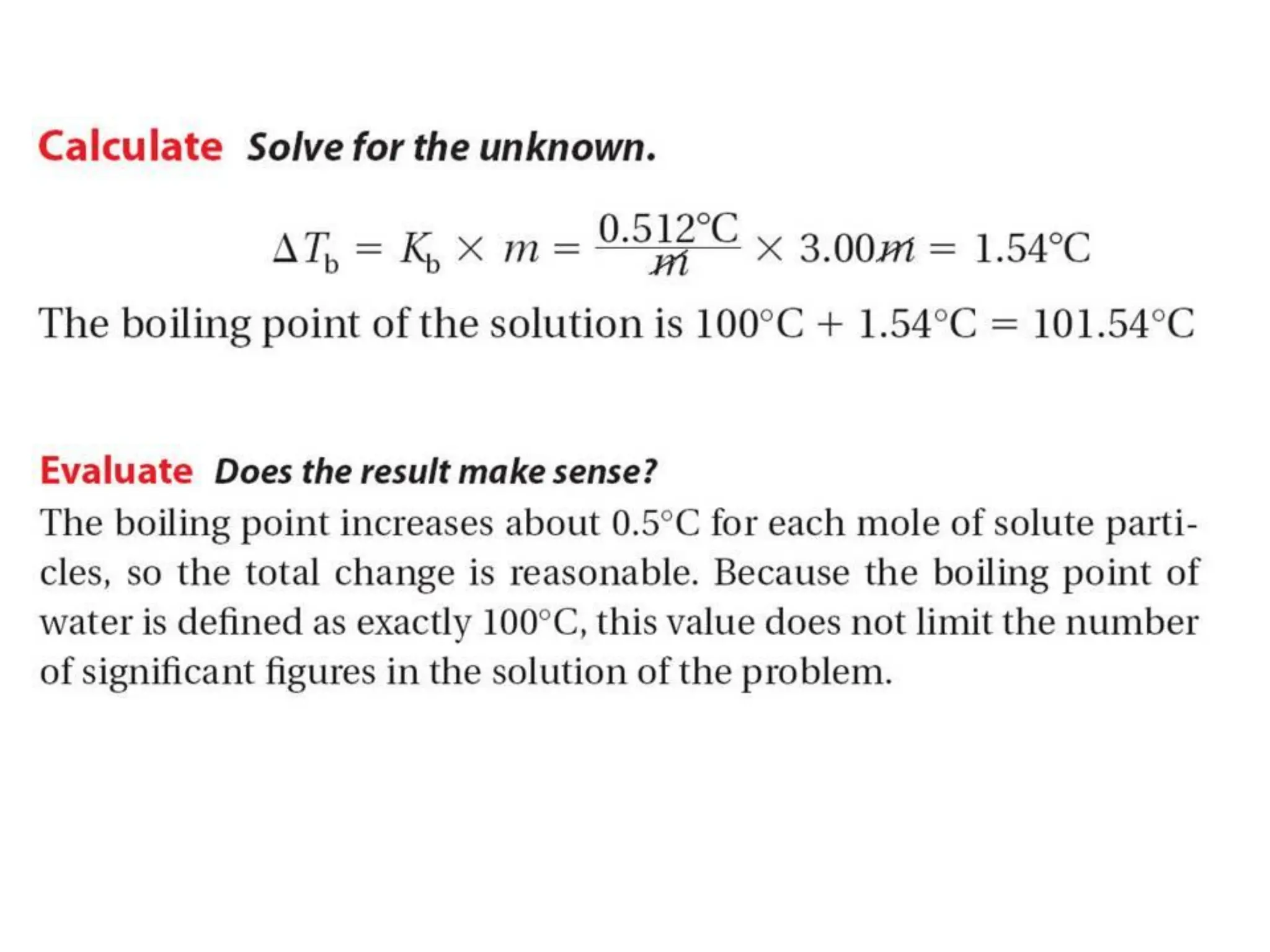
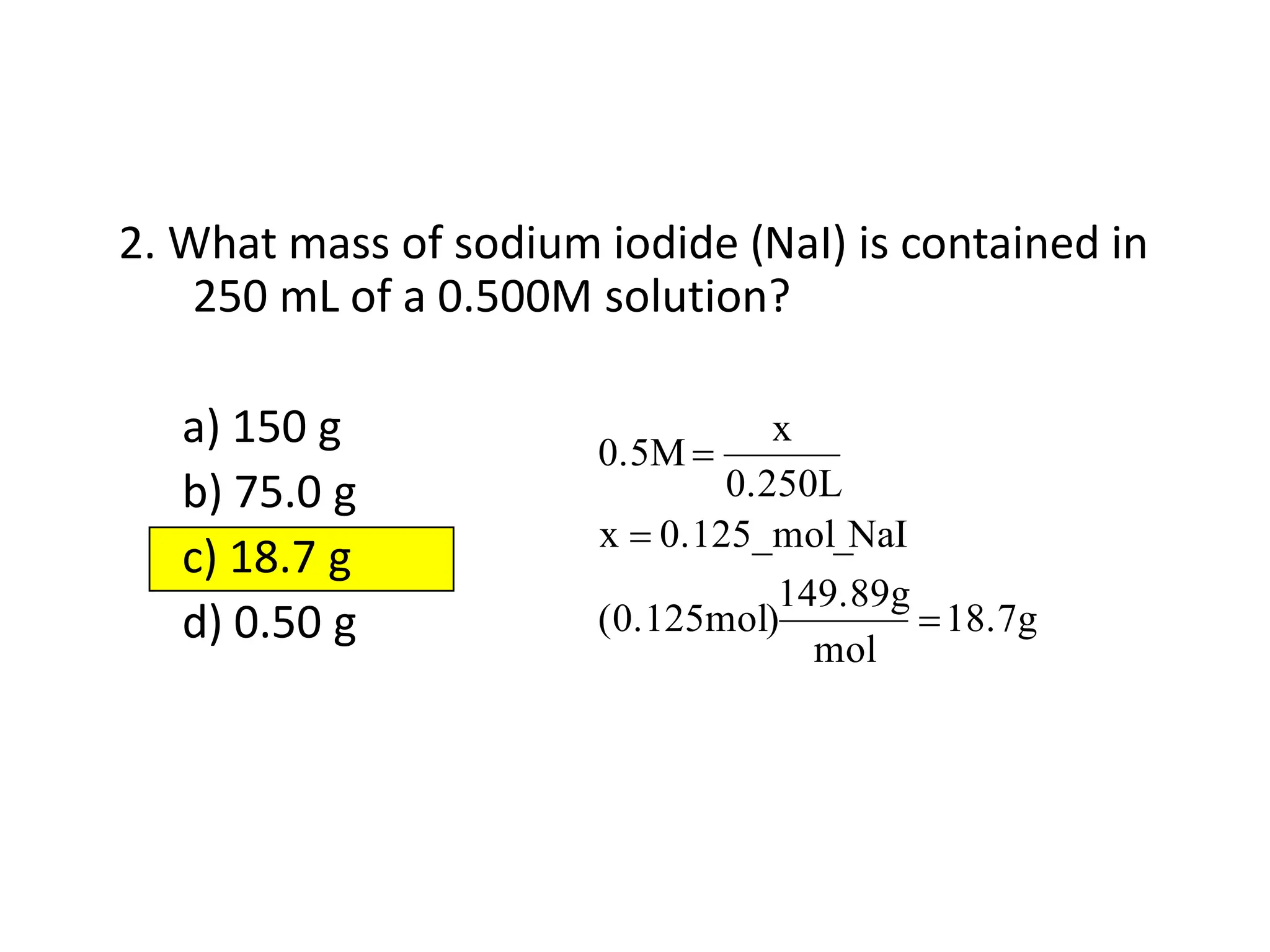The document discusses concentration of solutions and molarity. It defines molarity as the number of moles of solute dissolved in 1 liter of solution. It provides examples of calculating molarity based on moles of solute and volume of solution. It also discusses making dilute solutions by taking an initial volume of a concentrated stock solution and diluting it with solvent to a final volume. Dilution does not change the total moles of solute.