Embed presentation
Download to read offline

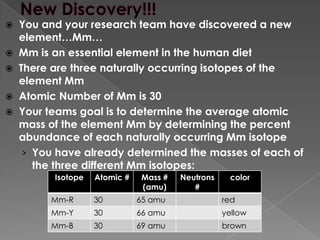
![CalculationsPercent Abundance of isotope Mm-R=(Total # of Mm-R in nature/ Total # of all Mm-isotopes in nature) x 100%Percent Abundance of isotope Mm-B=(Total # of Mm-B in nature/ Total # of all Mm-isotopes in nature) x 100%Percent Abundance of isotope Mm-Y=(Total # of Mm-Y in nature/ Total # of all Mm-isotopes in nature) x 100%Mass Contribution of each isotope:(amu of Mm isotope) x (percent abundance of Mm isotope in nature)Average Atomic Mass= [(mass contribution of Mm-R) + (mass contr. of Mm-B) + (mass contr. of Mm-Y)] Show ALL CalculationsTake Pictures of Set upRecord all procedures](https://image.slidesharecdn.com/mmisotopediscoverylab-110922101739-phpapp02/85/Mm-isotope-discovery-lab-3-320.jpg)


A new element, Mm, has been discovered with an atomic number of 30. Mm has three naturally occurring isotopes and is an essential element in the human diet. The research team's goal is to determine the average atomic mass of Mm by finding the percent abundance of each isotope and calculating the mass contribution of each one. They have already determined the masses of the three Mm isotopes and now need to perform calculations to find the percent abundances and average atomic mass.


![CalculationsPercent Abundance of isotope Mm-R=(Total # of Mm-R in nature/ Total # of all Mm-isotopes in nature) x 100%Percent Abundance of isotope Mm-B=(Total # of Mm-B in nature/ Total # of all Mm-isotopes in nature) x 100%Percent Abundance of isotope Mm-Y=(Total # of Mm-Y in nature/ Total # of all Mm-isotopes in nature) x 100%Mass Contribution of each isotope:(amu of Mm isotope) x (percent abundance of Mm isotope in nature)Average Atomic Mass= [(mass contribution of Mm-R) + (mass contr. of Mm-B) + (mass contr. of Mm-Y)] Show ALL CalculationsTake Pictures of Set upRecord all procedures](https://image.slidesharecdn.com/mmisotopediscoverylab-110922101739-phpapp02/85/Mm-isotope-discovery-lab-3-320.jpg)
