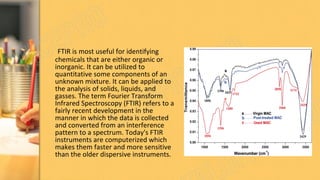
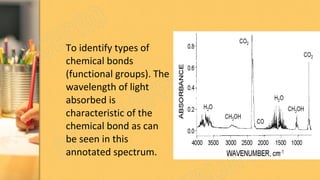
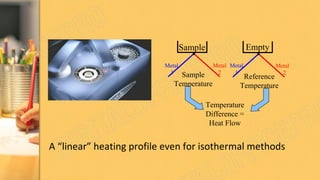
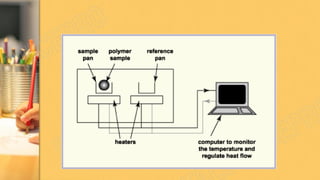
There are three main methods to determine dye chemistry: Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), and infrared (IR) spectroscopy. FTIR identifies chemical bonds by detecting molecular absorption of infrared radiation. DSC measures heat flows involved in material transitions as a function of temperature. IR spectroscopy identifies functional groups based on their absorption of infrared radiation frequencies. An example analyzed the functional groups of a commercially available inkjet printing reactive dye using IR spectroscopy to understand its chemical structure.