Merck presented at the 2016 ASCO conference on their oncology strategy and KEYTRUDA program. Key points include:
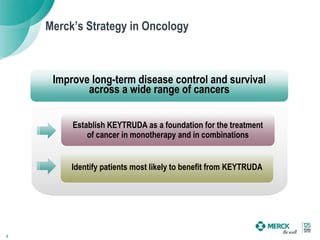
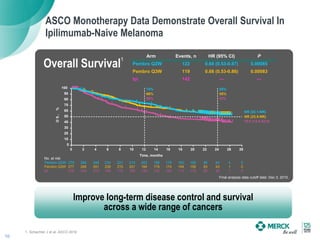
1) Merck's strategy is to identify patients most likely to benefit from KEYTRUDA, establish KEYTRUDA as a foundation for cancer treatment in monotherapy and combinations, and improve long-term disease control and survival across cancers.
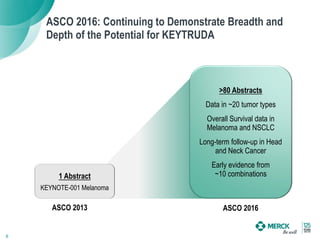
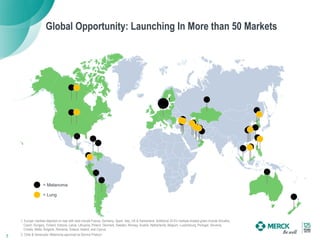
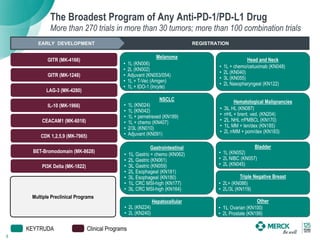
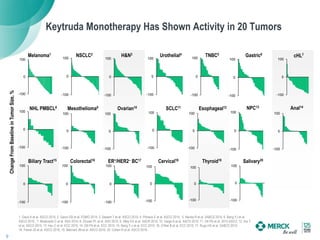
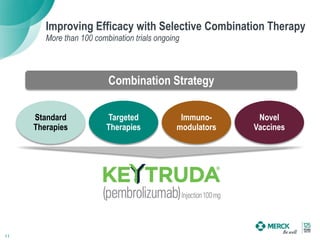
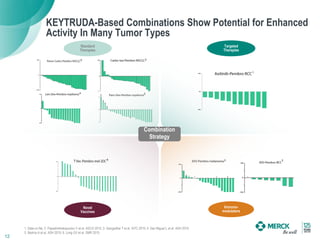
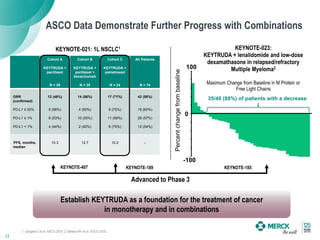
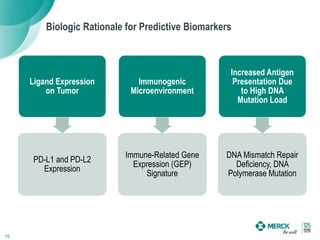
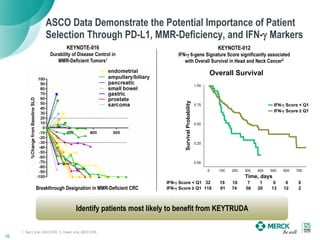
2) KEYTRUDA has shown clinical activity in over 20 tumor types and has over 30 ongoing registration-enabling studies and 100 combination trials. Data at ASCO 2016 showed further progress in combinations and predictive biomarkers.
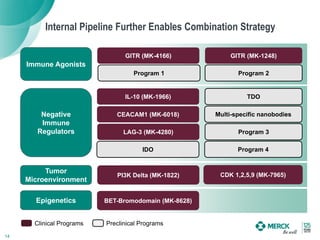
3) Merck is pursuing a combination strategy with KEYTRUDA and targeted therapies, immunomodulators