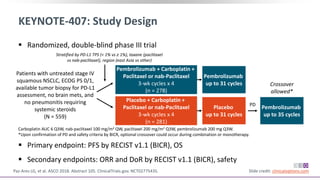
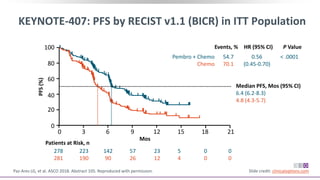
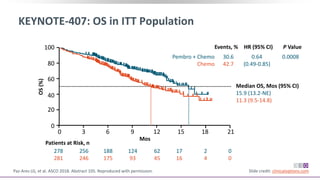
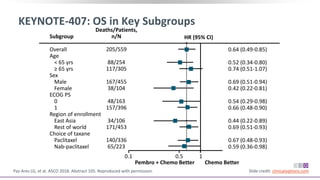
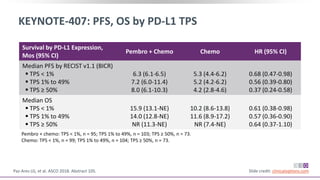
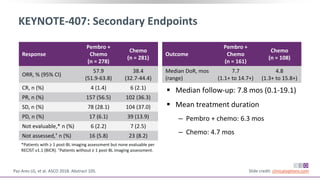
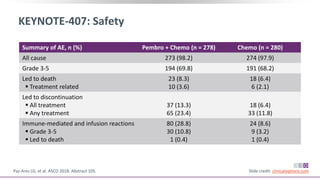
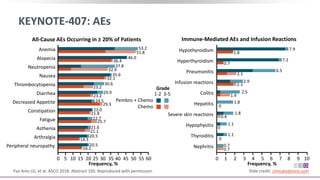
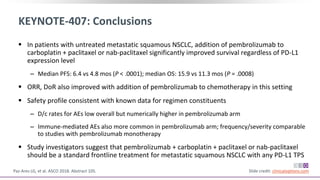
The keynote-407 phase III trial evaluated the addition of pembrolizumab to carboplatin and paclitaxel/nab-paclitaxel in untreated stage IV squamous NSCLC patients, demonstrating significant improvements in progression-free survival (PFS) and overall survival (OS) compared to chemotherapy alone. The results showed a median PFS of 6.4 months versus 4.8 months, and a median OS of 15.9 months versus 11.3 months, regardless of PD-L1 expression levels. Despite a somewhat higher rate of adverse events in the pembrolizumab group, the safety profile was consistent with known data, leading investigators to recommend this combination as a standard frontline treatment for this patient population.

![Carboplatin + Paclitaxel/nab-Paclitaxel ±
Pembrolizumab in NSCLC (KEYNOTE-407): Background
Pembrolizumab: monoclonal antibody targeting PD-1
‒ Significantly improved OS and PFS with fewer AEs vs platinum-based
chemotherapy in patients with untreated advanced squamous or
nonsquamous PD-L1–positive NSCLC[1]
‒ Significantly improved OS and PFS when added to pemetrexed + a
platinum-based agent in patients with untreated metastatic
nonsquamous NSCLC, regardless of PD-L1 status[2]
Current prespecified second interim analysis evaluates efficacy and
safety of pembrolizumab addition to platinum-based chemotherapy in
patients with untreated metastatic squamous NSCLC[3]
1. Reck M, et al. N Engl J Med. 2016;375:1823-1833. 2. Gandhi L, et al. N Engl J Med.
2018;378:2078-2092. 3. Paz-Ares LG, et al. ASCO 2018. Abstract 105. Slide credit: clinicaloptions.com](https://image.slidesharecdn.com/ccokeynote407-190803041604/85/Cco-keynote-407-2-320.jpg)