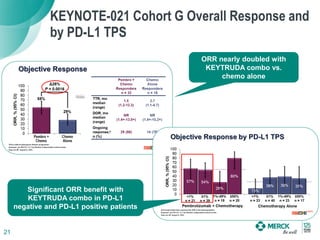
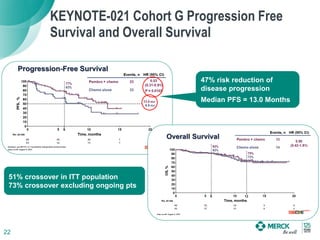
The KEYNOTE-052 study evaluated pembrolizumab monotherapy in the first-line treatment of advanced urothelial carcinoma in patients ineligible for cisplatin-based chemotherapy. In the planned interim analysis of the first 100 patients, the objective response rate was 24%, including a complete response in 6% of patients. Fifteen percent of patients experienced stable disease as their best response. The study aims to determine the optimal PD-L1 expression cut point for predicting response to pembrolizumab.