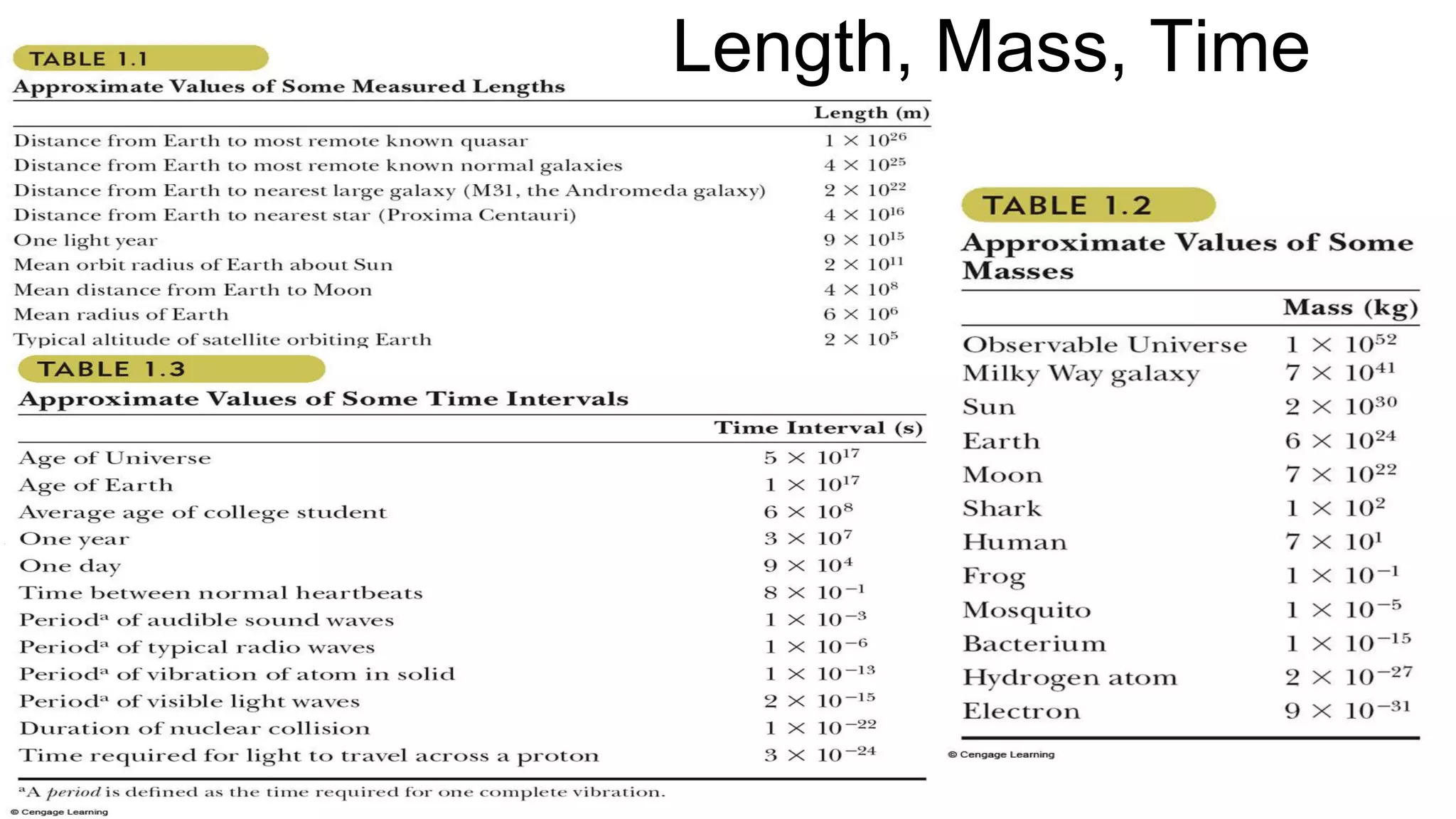
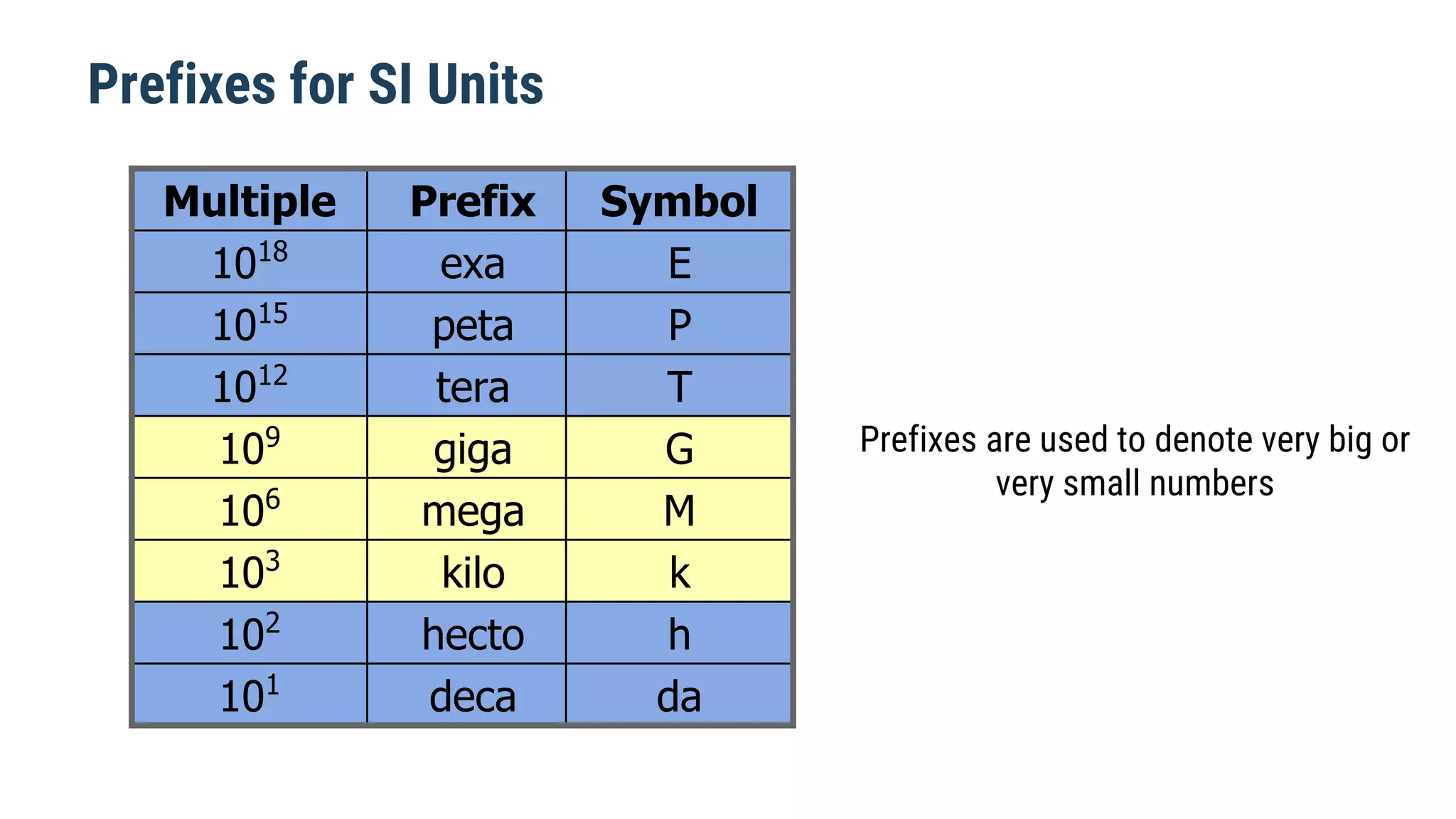
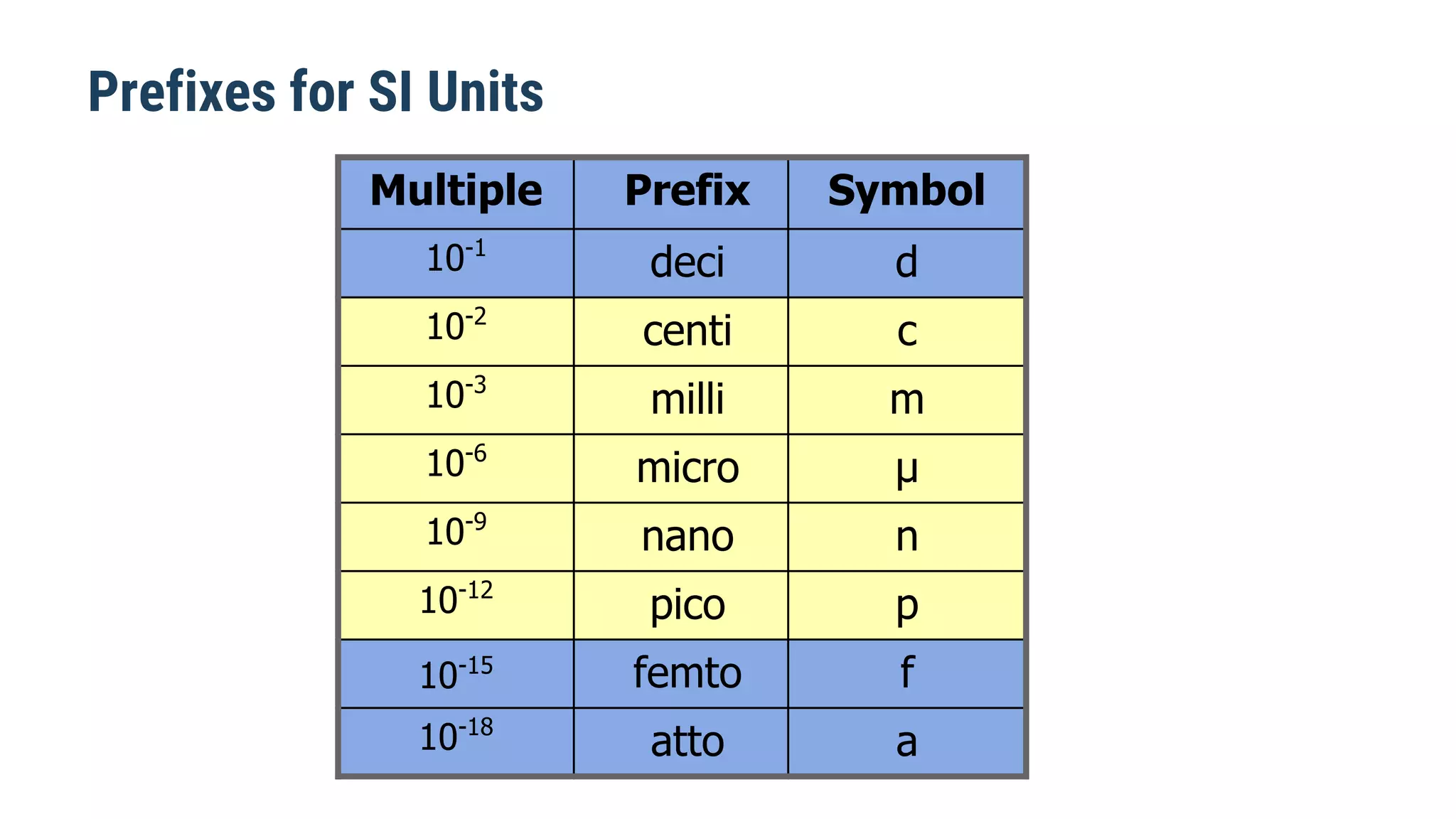
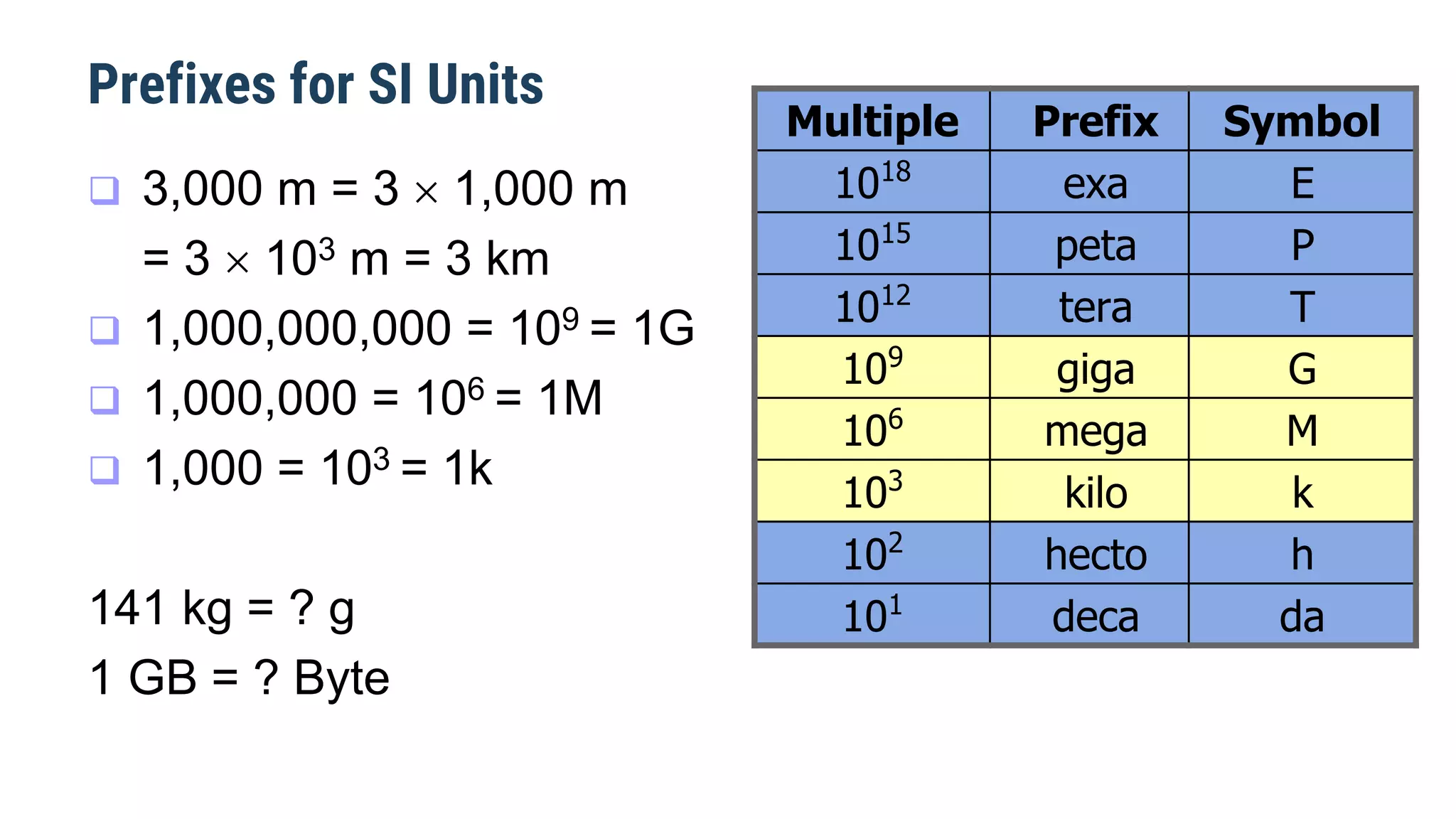
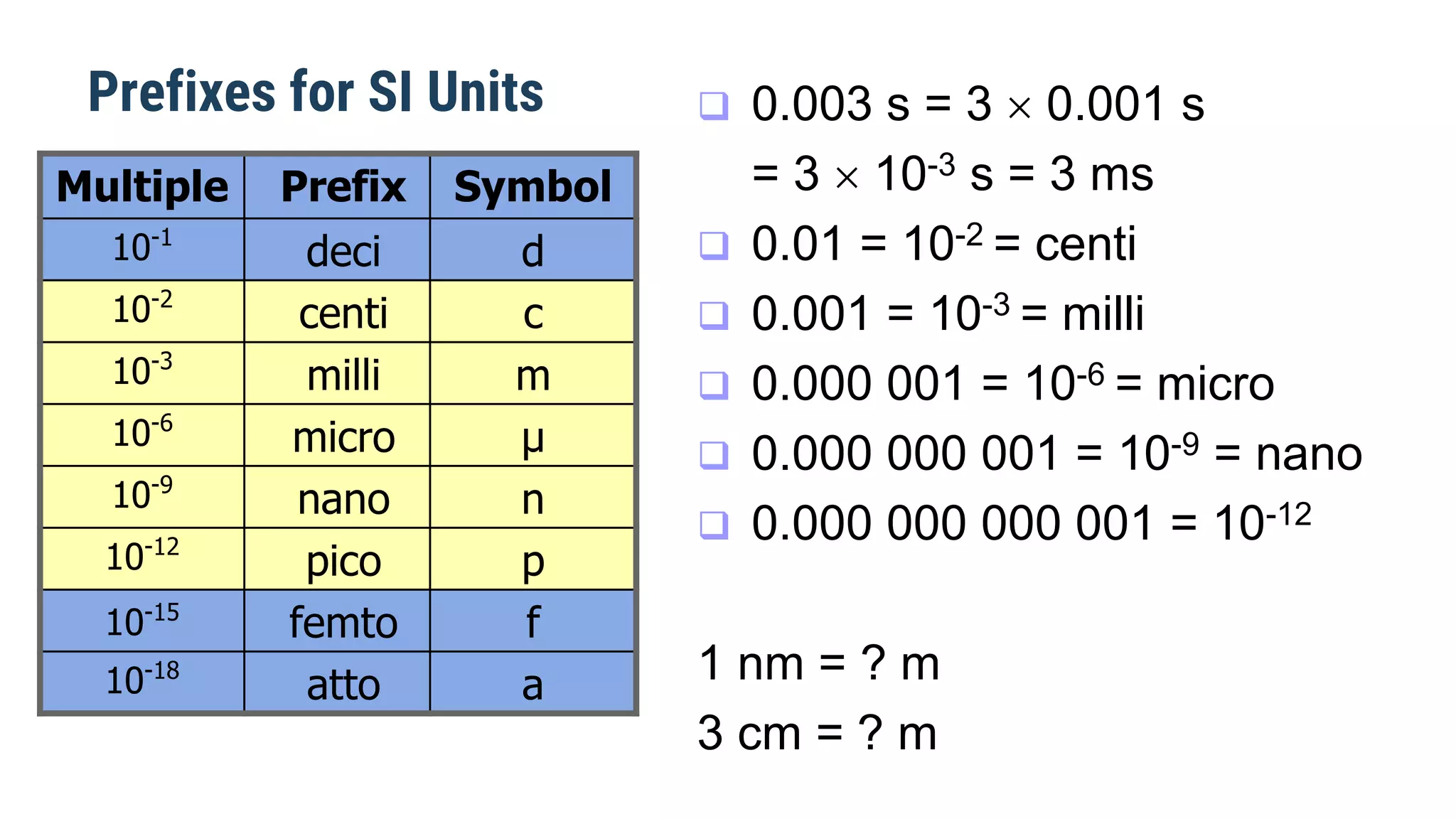
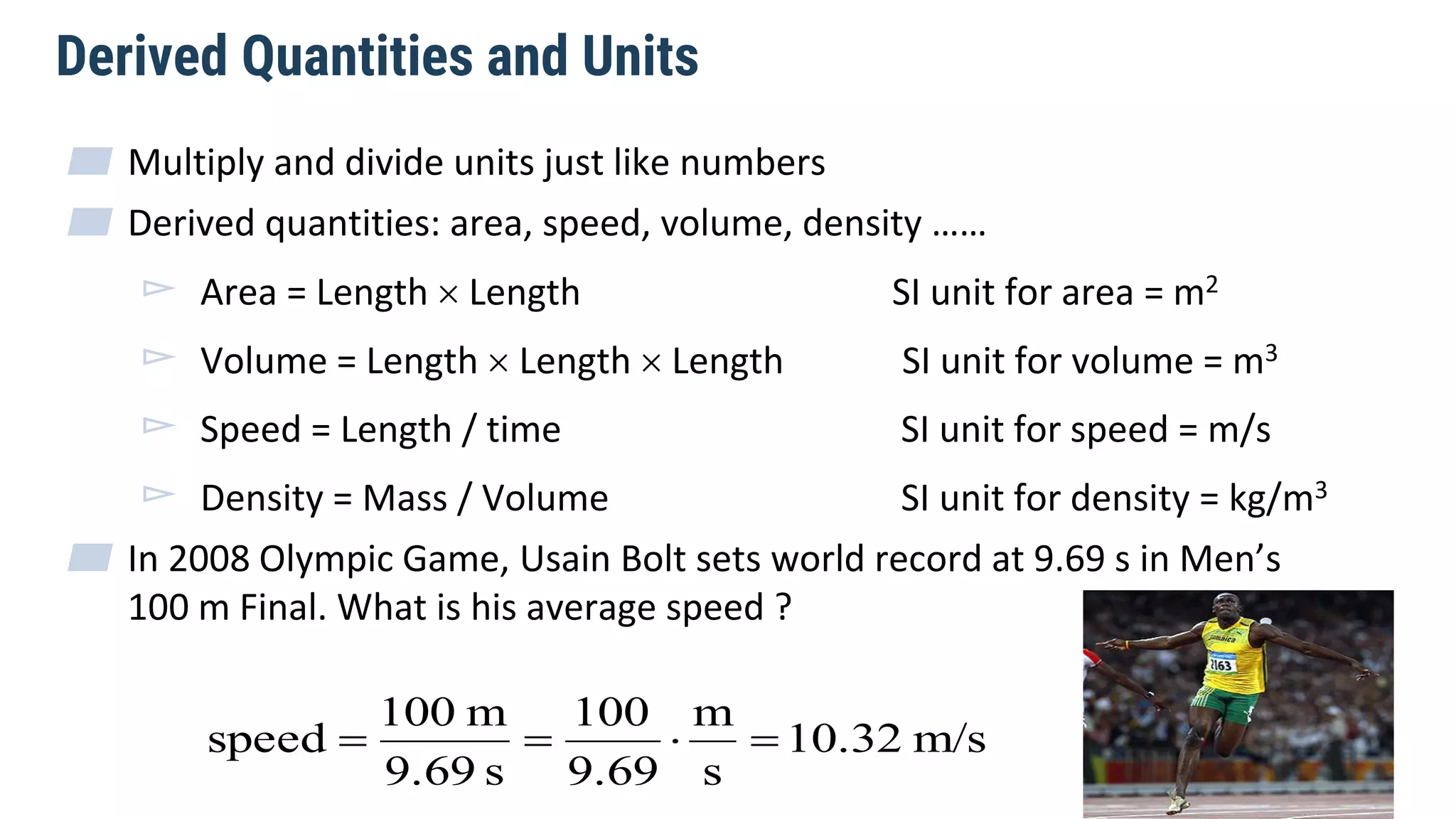
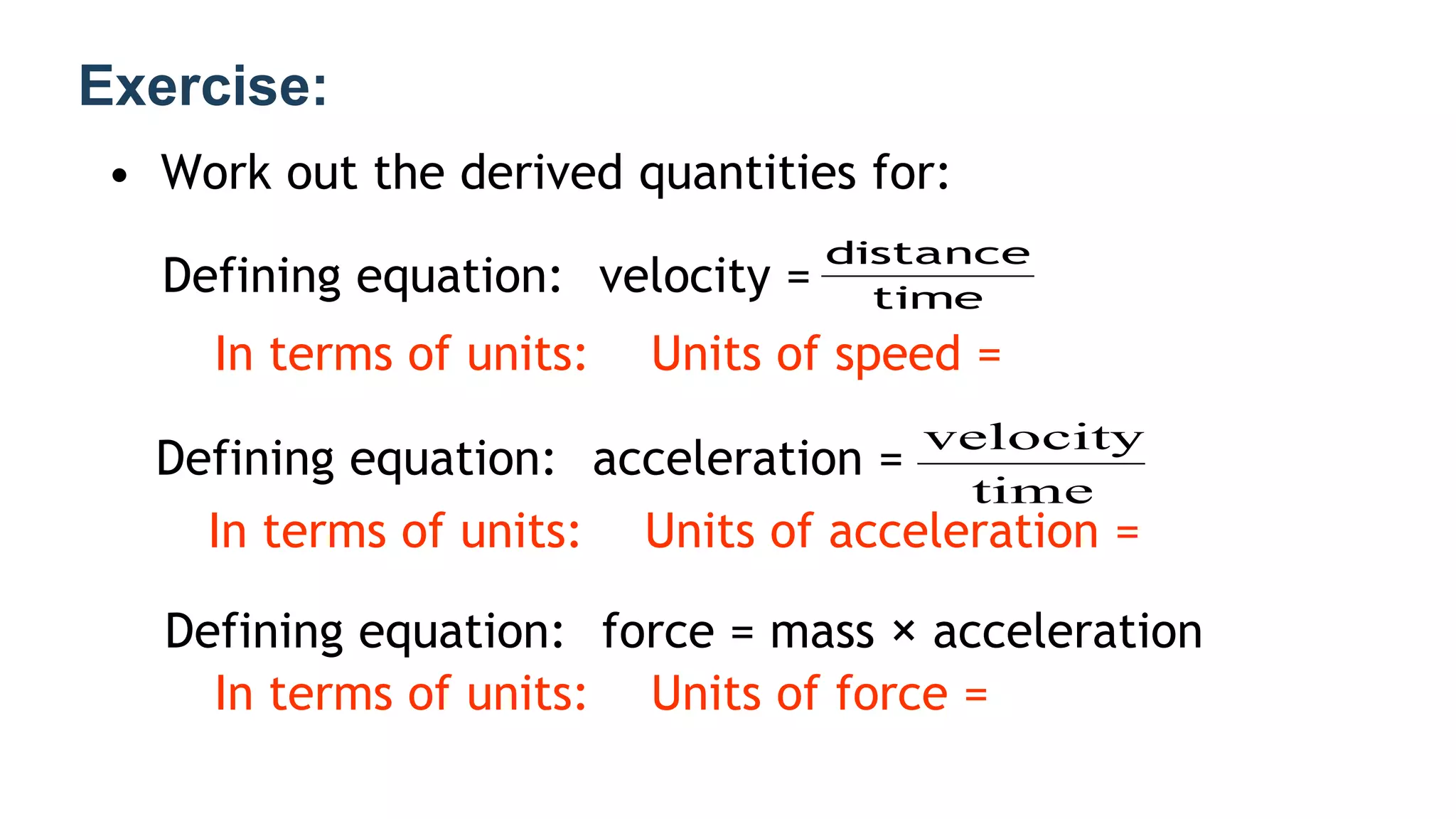
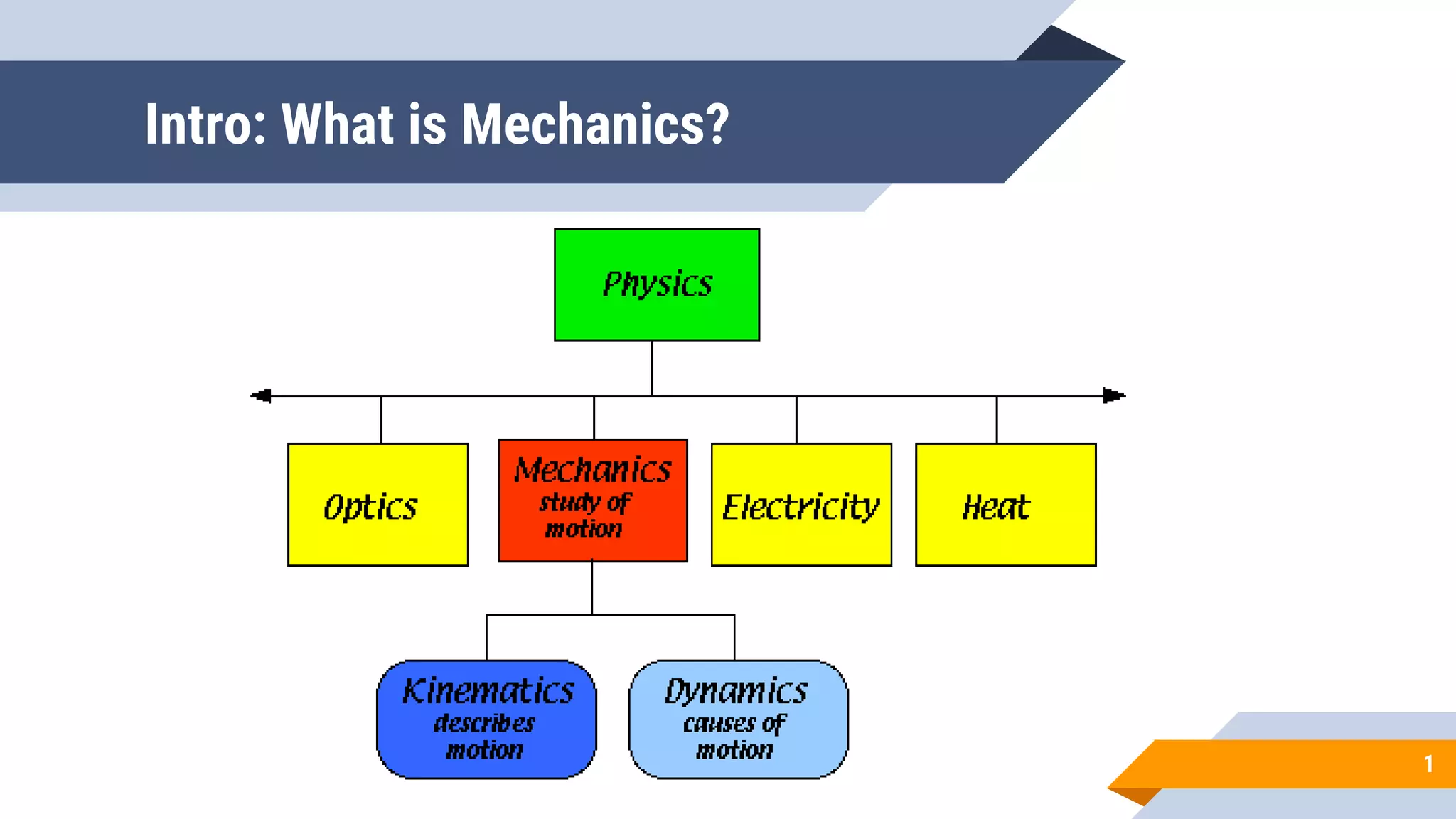
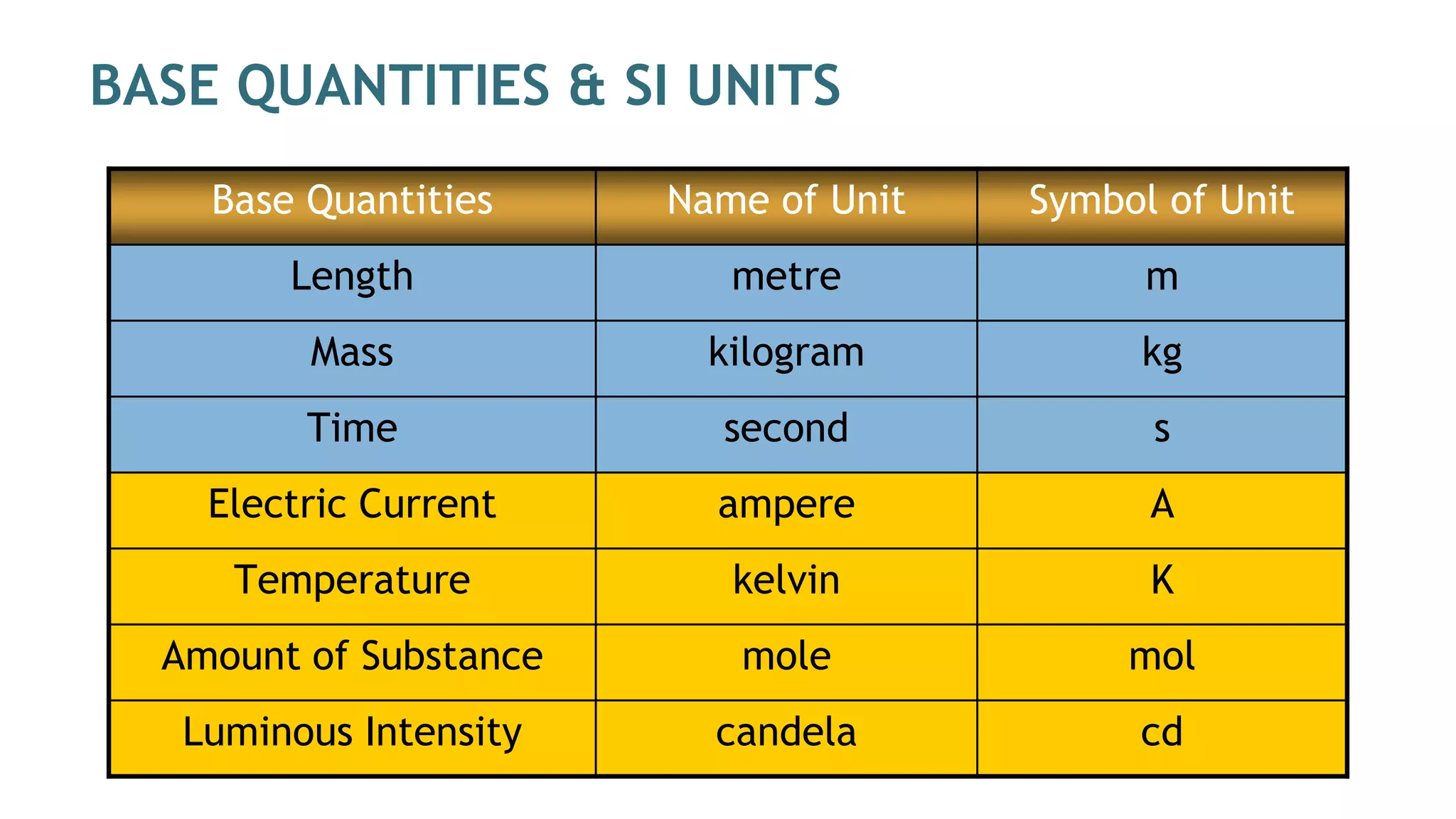
Mechanics is the branch of physics dealing with the study of motion. It can be divided into kinematics, which deals with describing motions, and dynamics, which deals with the causes of motion. The International System of Units (SI) provides standardized units for measuring physical quantities. The three base SI units are the meter (length), kilogram (mass), and second (time). Derived quantities like area, speed, and density can be calculated using the appropriate mathematical operations and combinations of base units. Problem solving in physics requires quantifying observations with standardized units.





![SI Length Unit: Meter
▰ Length describe the quantity of space such width, height or
distance
▰ Symbol [L]
▰ Current Definition of 1 Meter: the distance traveled by light
in vacuum during a time of 1/299,792,458 second.
▰ 1 Meter = about 3.28 ft
▰ 1 km = 1000 m, 1 cm = 1/100 m, 1 mm = 1/1000 m](https://image.slidesharecdn.com/mechanicsweek1units-200328071333/75/Mechanics-Chapter-1-units-base-derived-quantities-10-2048.jpg)
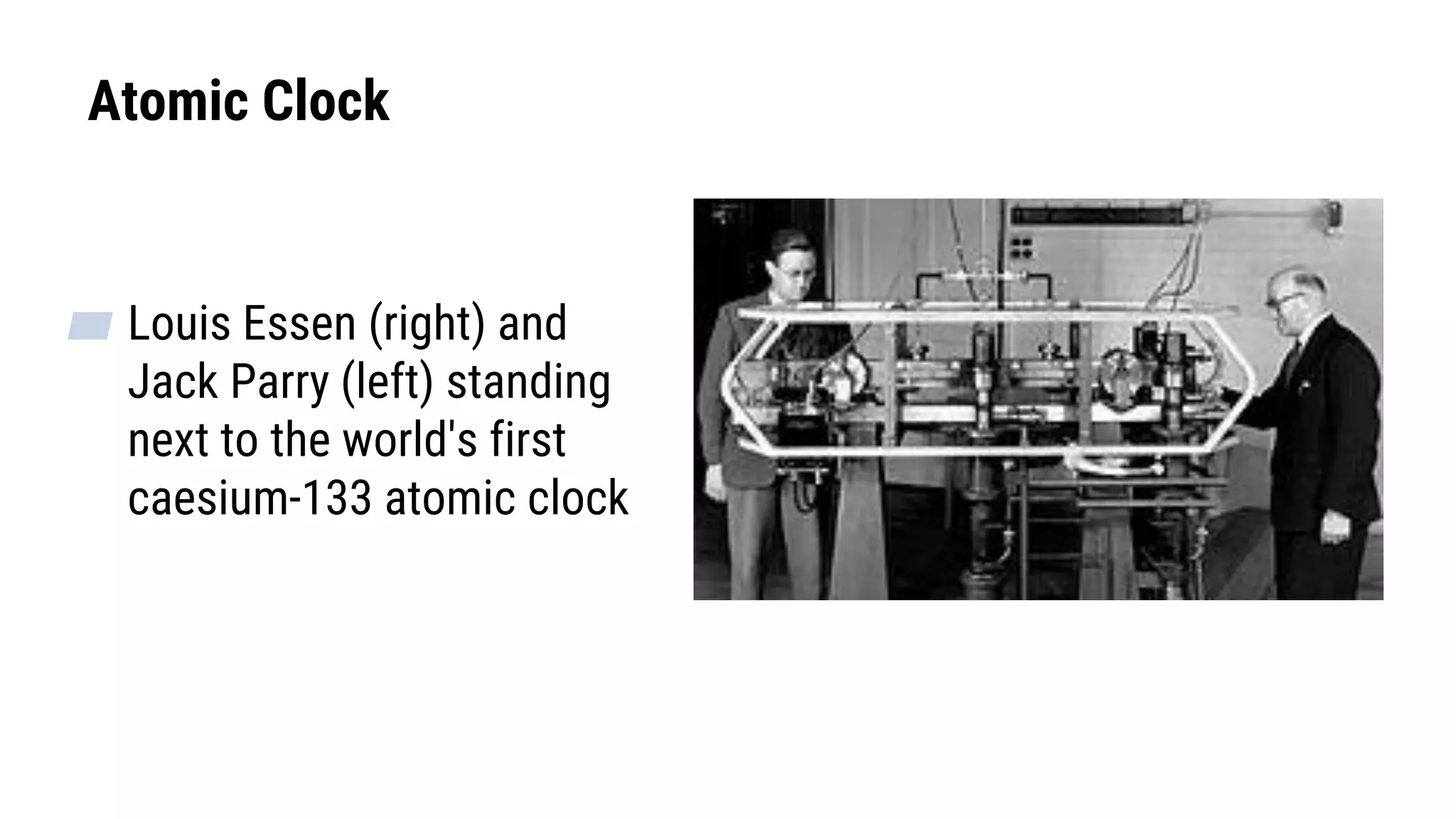
![SI Time Unit: Second
▰ Time describes the flow of the universe from the past through
the present into the future. In physics, usually mean a quantity
of time in seconds, such as 35s.
▰ Symbol [T]
▰ The solar clock was originally used to define the second.
▰ 1 Second is defined in terms of an “atomic clock”– time taken
for 9,192,631,770 oscillations of the light emitted by a
cesium-133 atom.](https://image.slidesharecdn.com/mechanicsweek1units-200328071333/75/Mechanics-Chapter-1-units-base-derived-quantities-11-2048.jpg)
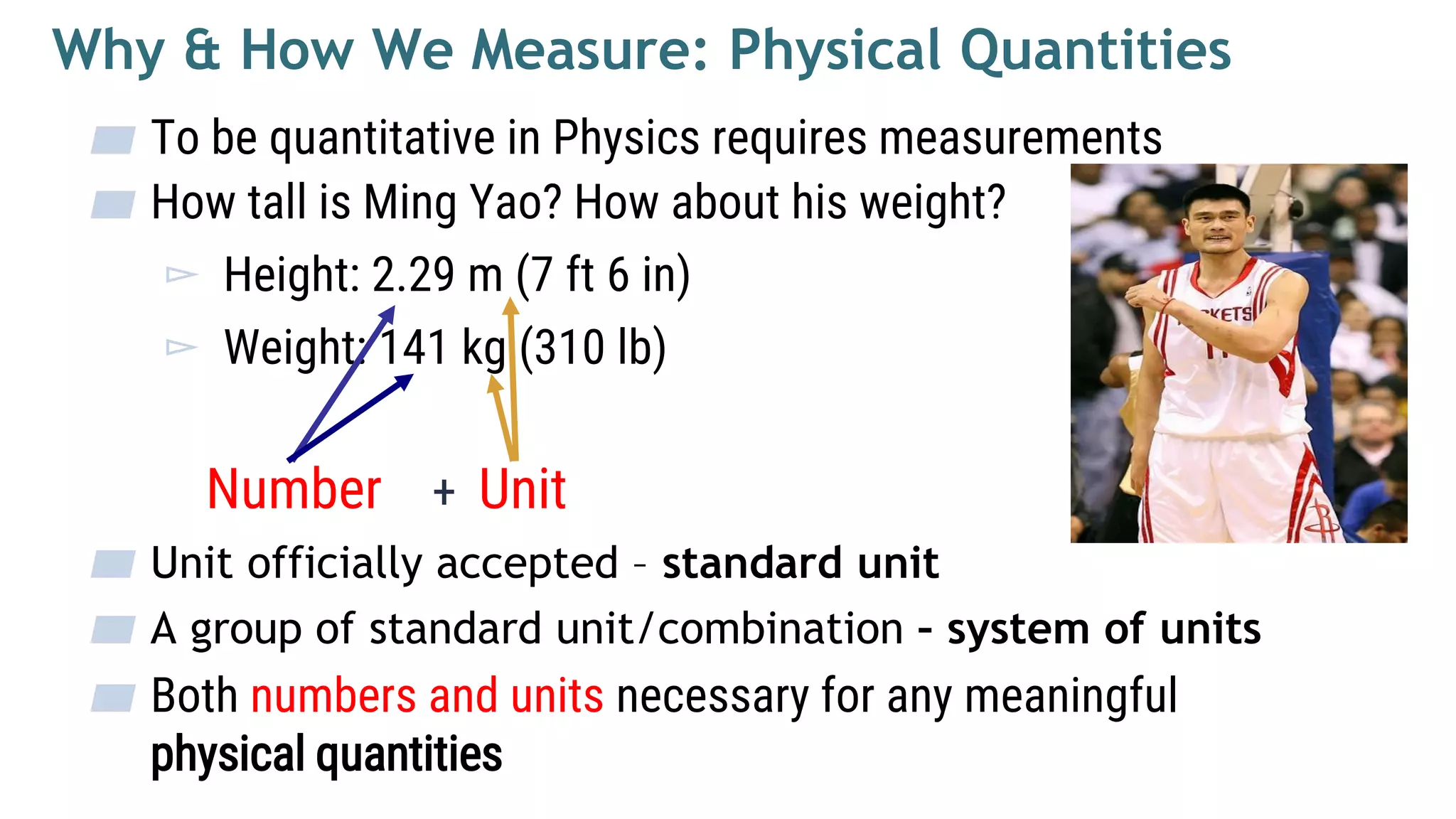
![SI Mass Unit: Kilogram
▰ Mass describes the quantity of matter
▰ Symbol [M]
▰ 1 Kilogram – the mass of a specific platinum-
iridium alloy kept at International Bureau of
Weights and Measures near Paris. Copies are
kept in many other countries.
▰ Yao Ming is 141 kg, equivalent to weight of 141
pieces of the alloy cylinder.](https://image.slidesharecdn.com/mechanicsweek1units-200328071333/75/Mechanics-Chapter-1-units-base-derived-quantities-13-2048.jpg)