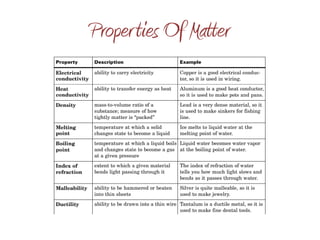
Matter exists in three states - solid, liquid, and gas. Physical changes alter a substance's state or form without changing its chemical makeup, while chemical changes create new substances. Physical properties like hardness, color, and melting point can be observed without changing a substance's identity, whereas chemical properties involve chemical reactions that alter a substance's identity.