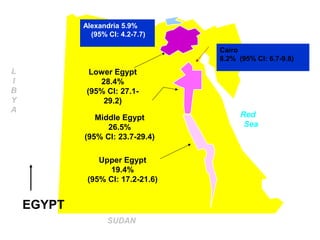
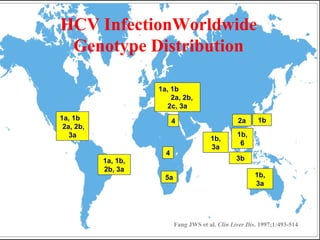
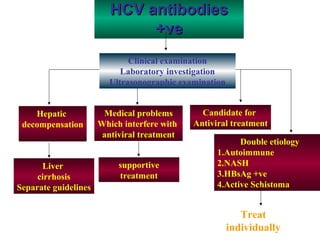
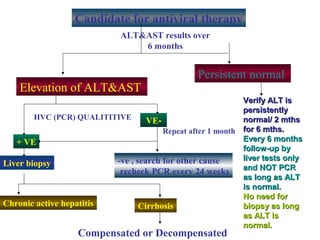
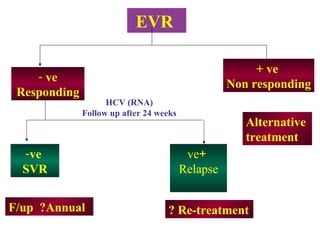
This document summarizes the key guidelines from the 2008 Egyptian-Italian consensus meeting on the management of hepatitis C virus (HCV) infection. Some of the main points covered include:
1) Liver biopsy is still recommended before HCV treatment to assess fibrosis level.
2) Fibroscan and laboratory markers alone cannot replace liver biopsy.
3) Genotyping is only necessary for patients who travel abroad or are treatment failures.
4) There is no upper age limit for HCV treatment. Pegylated interferon can be used in children ages 5 years old.
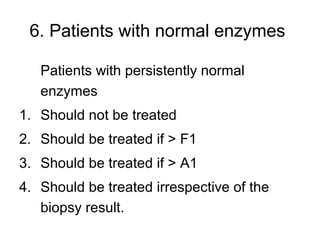
5) Patients with persistently normal liver enzymes should be treated if fibrosis is F1 or above.
6) Patients with compens