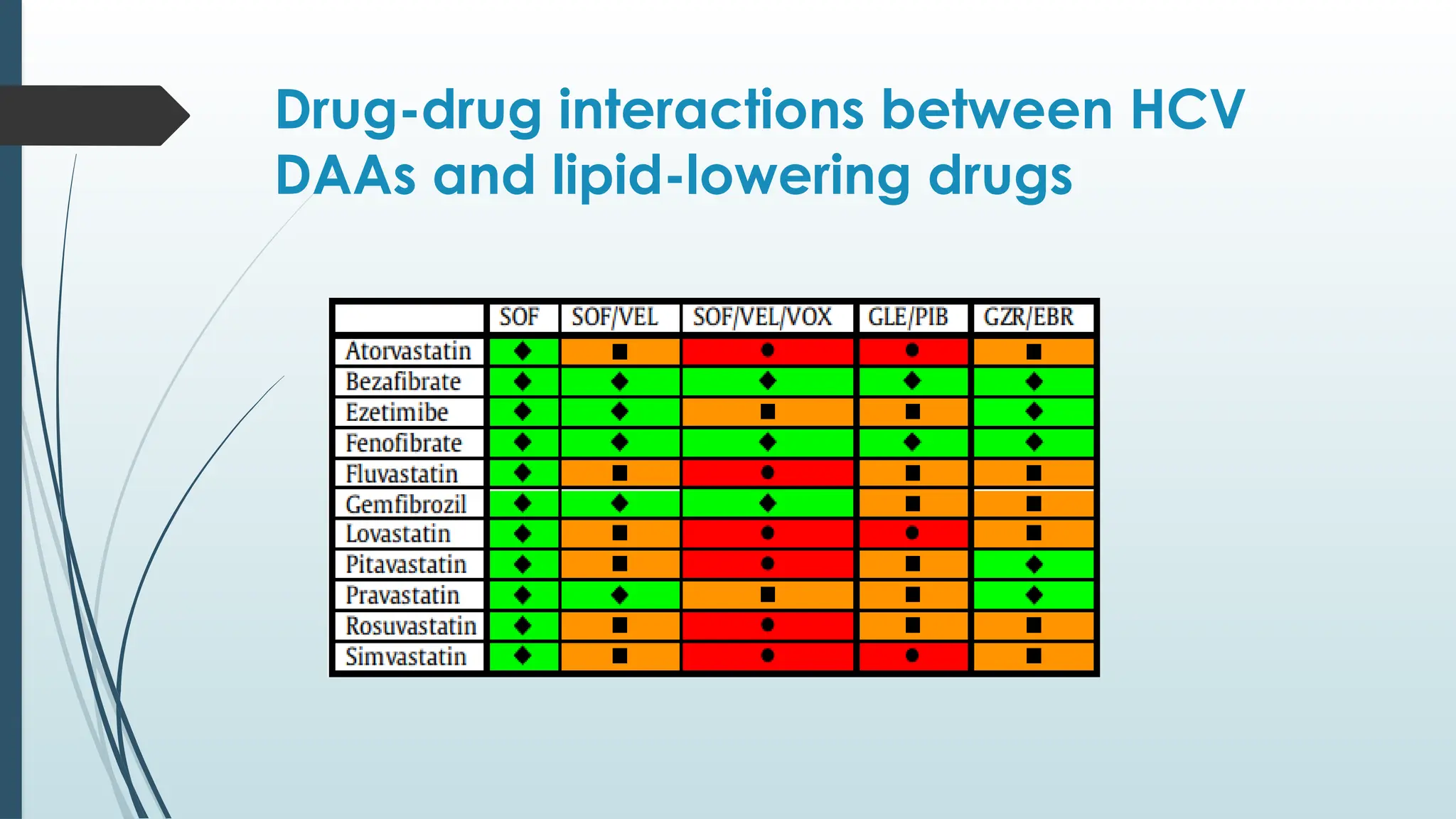
This document outlines the recommendations for managing hepatitis C (HCV) based on the latest guidelines, highlighting the epidemiology, treatment options, and assessment methods for liver fibrosis and cirrhosis. It emphasizes the importance of non-invasive methods for fibrosis assessment, the use of direct-acting antivirals (DAAs) for treatment, and specific considerations for treating special populations such as patients with co-infections or pregnant women. The primary goal of HCV therapy is to achieve a sustained virologic response (SVR) to prevent liver-related complications and improve patient quality of life.