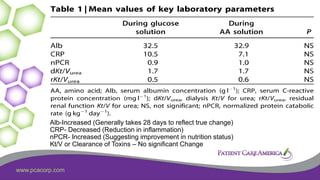
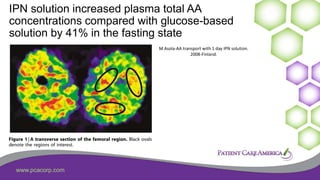
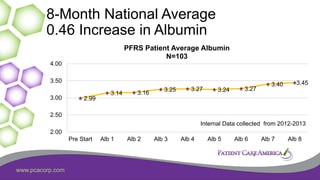
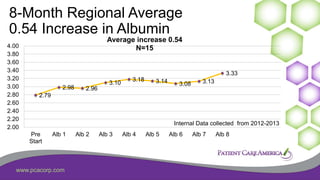
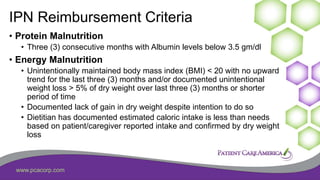
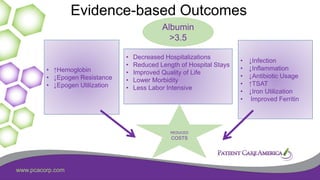
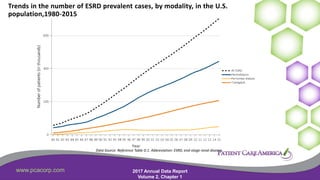
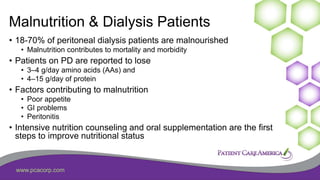
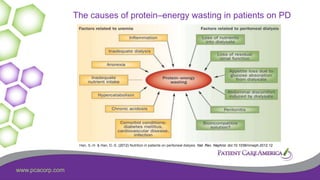
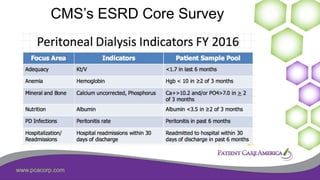
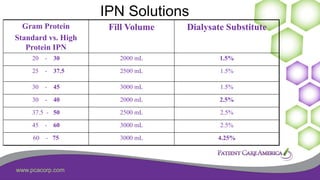
This document discusses the prevalence of malnutrition among peritoneal dialysis patients and explores the benefits of intraperitoneal nutrition (IPN) as an intervention. It highlights the causes of malnutrition, methods to improve nutrition status, and the positive outcomes associated with IPN, such as improved protein retention and better overall health. The document also includes various research studies and empirical evidence supporting IPN's effectiveness in enhancing patient care.
















![www.pcacorp.com
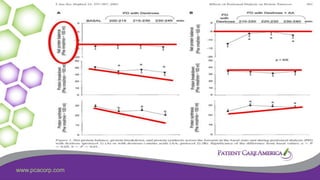
1) Tjiong, IPN Promotes Protein
Synthesis – 2007
• 12 Continuous ambulatory peritoneal dialysis (CAPD)
• AA (Nutrineal 1.1%) plus G (Physioneal l.36 to 3.86%)
versus G only as control dialysate.
• Using AA plus G dialysate, as compared with the control,
• rates of protein synthesis increased significantly (2.02 0.08
versus 1.94 0.07 mol leucine/kg per min [mean SEM]; P 0.039).](https://image.slidesharecdn.com/malnutritioninpd-200302200137/85/Malnutrition-in-Peritoneal-Dialysis-29-320.jpg)