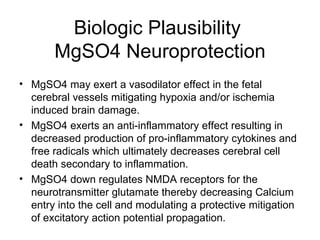
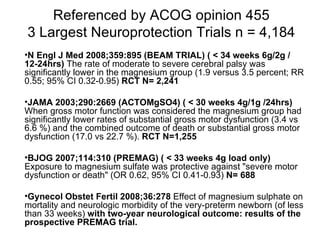
The document discusses evidence that magnesium sulfate administered to mothers at risk of preterm birth reduces the risk of cerebral palsy in infants. Several large randomized controlled trials and meta-analyses involving over 15,000 women found magnesium sulfate decreased the risk of cerebral palsy without increasing mortality. The number needed to treat to prevent one case of cerebral palsy is estimated at 63 women. The document also describes a modified magnesium sulfate neuroprotection protocol used at Riverside Hospital for imminent preterm deliveries between 23 and 33 weeks.


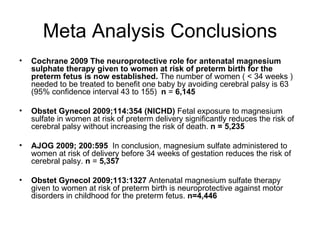

![DATA SUMMARY Arch Pediatr. 2011 Mar;18(3):324-30. It was shown in the Cochrane database and in 3 meta-analyses of 5 randomized trials: (Magpie (PIH), MagNet (Tocolysis/Neuroprotect), ActoMgSO [neuroprotection], PreMag [neuroprotection], and Beam [neuroprotection]) that prenatal magnesium sulfate given to mothers at risk of pre-term birth does not increase pediatric mortality and decreases the risk of cerebral palsy. Magnesium Sulfate exerts significant neuroprotective effects on the occurrence of cerebral palsy at 2 years of age (relative risk, 0.69; 95% CI, 0.54-0.87) Magnesium Sulfate decreased the risk of pediatric mortality and cerebral palsy (relative risk: 0.85; 95% confidence interval: 0.74-0.98). The number needed to treat (NTT) to prevent 1 case of cerebral palsy was 63 (95% CI, 39-172) and the NTT for an extra survivor free of cerebral palsy in the neuroprotection subgroup was 42 (95% CI, 22-357), justifying that magnesium sulfate should be justifying that magnesium sulfate should be discussed as a stand-alone treatment](https://image.slidesharecdn.com/magnesiumsulfate-120206113353-phpapp02/85/Magnesium-sulfate-7-320.jpg)


