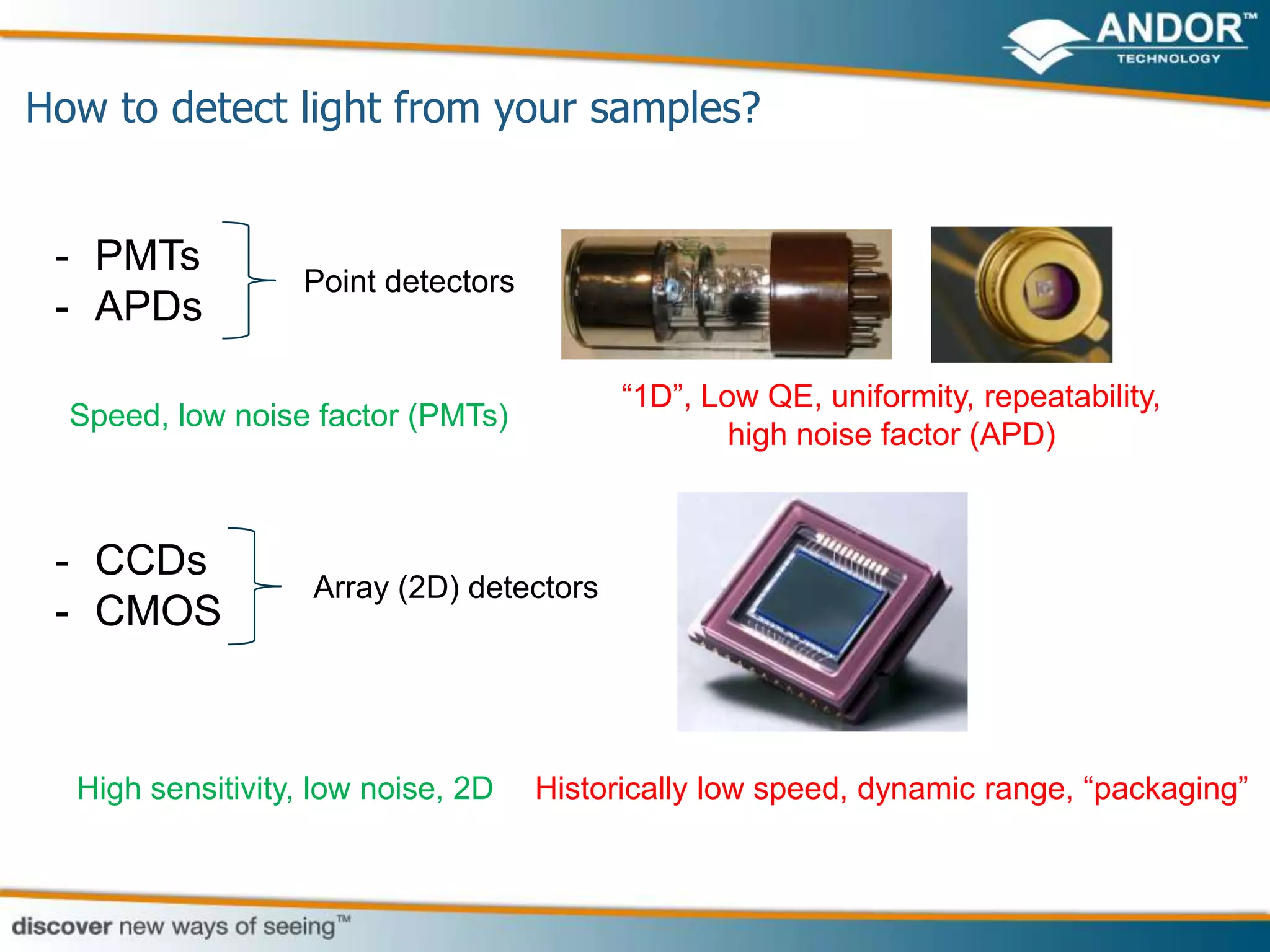
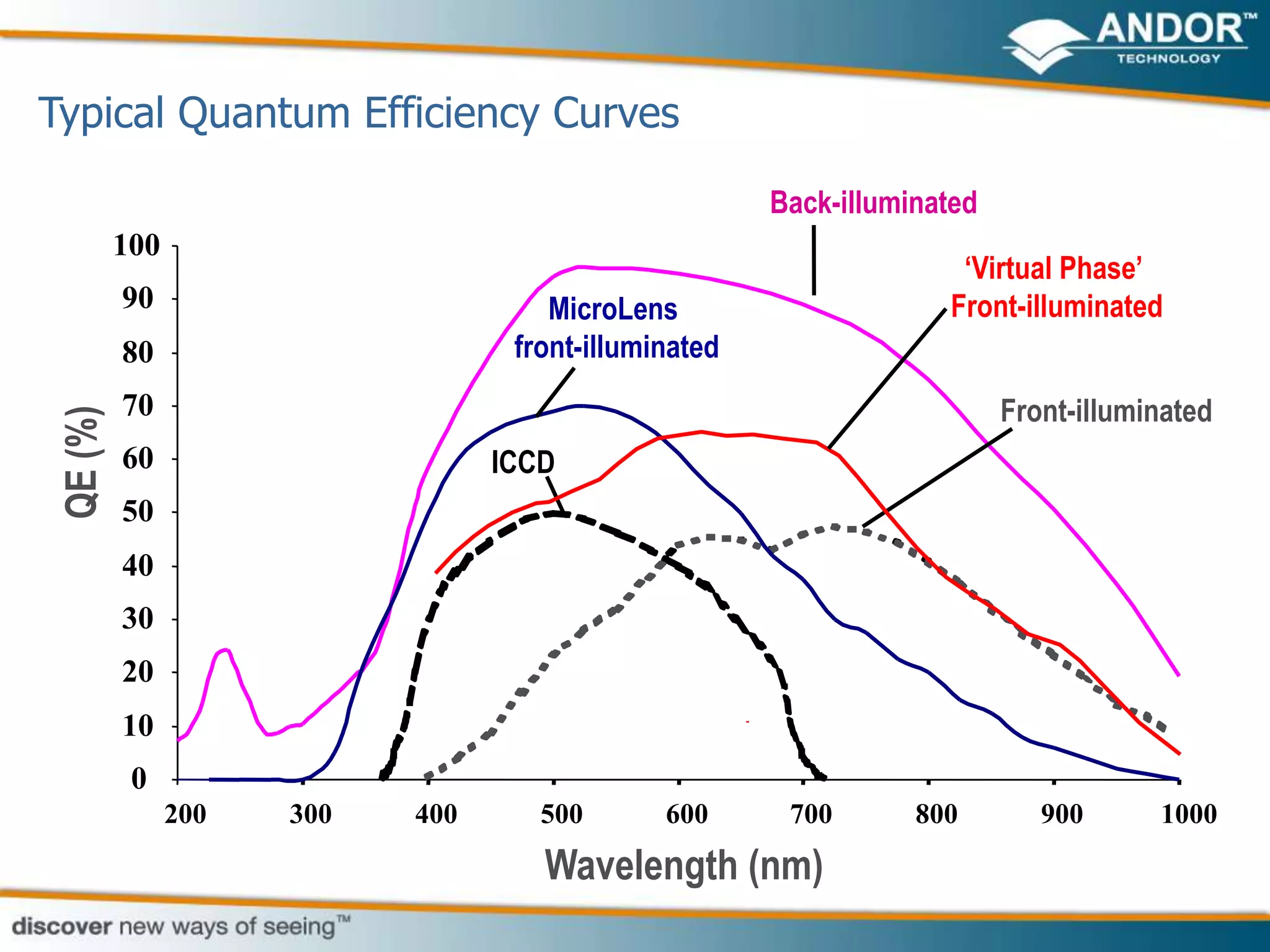
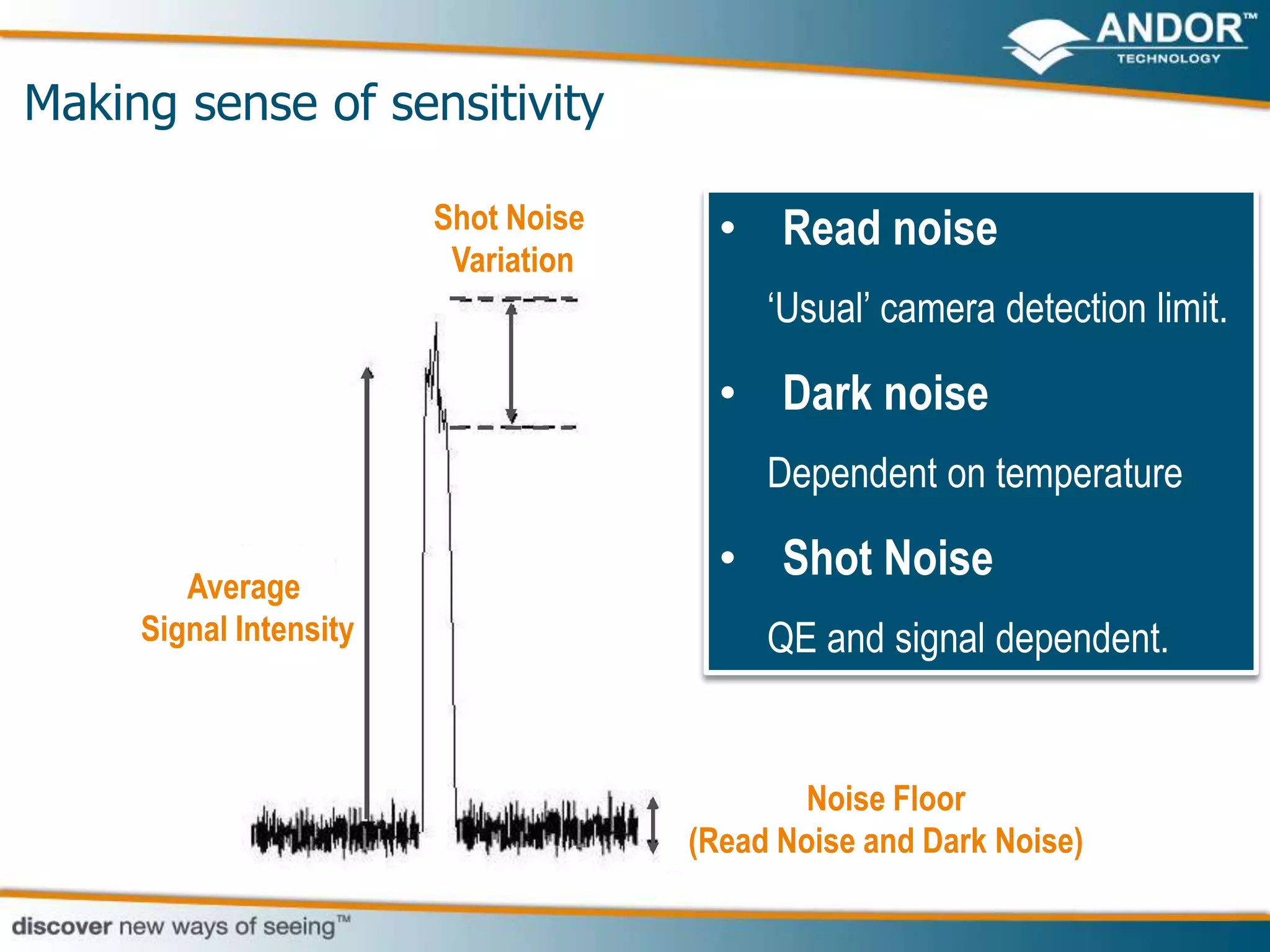
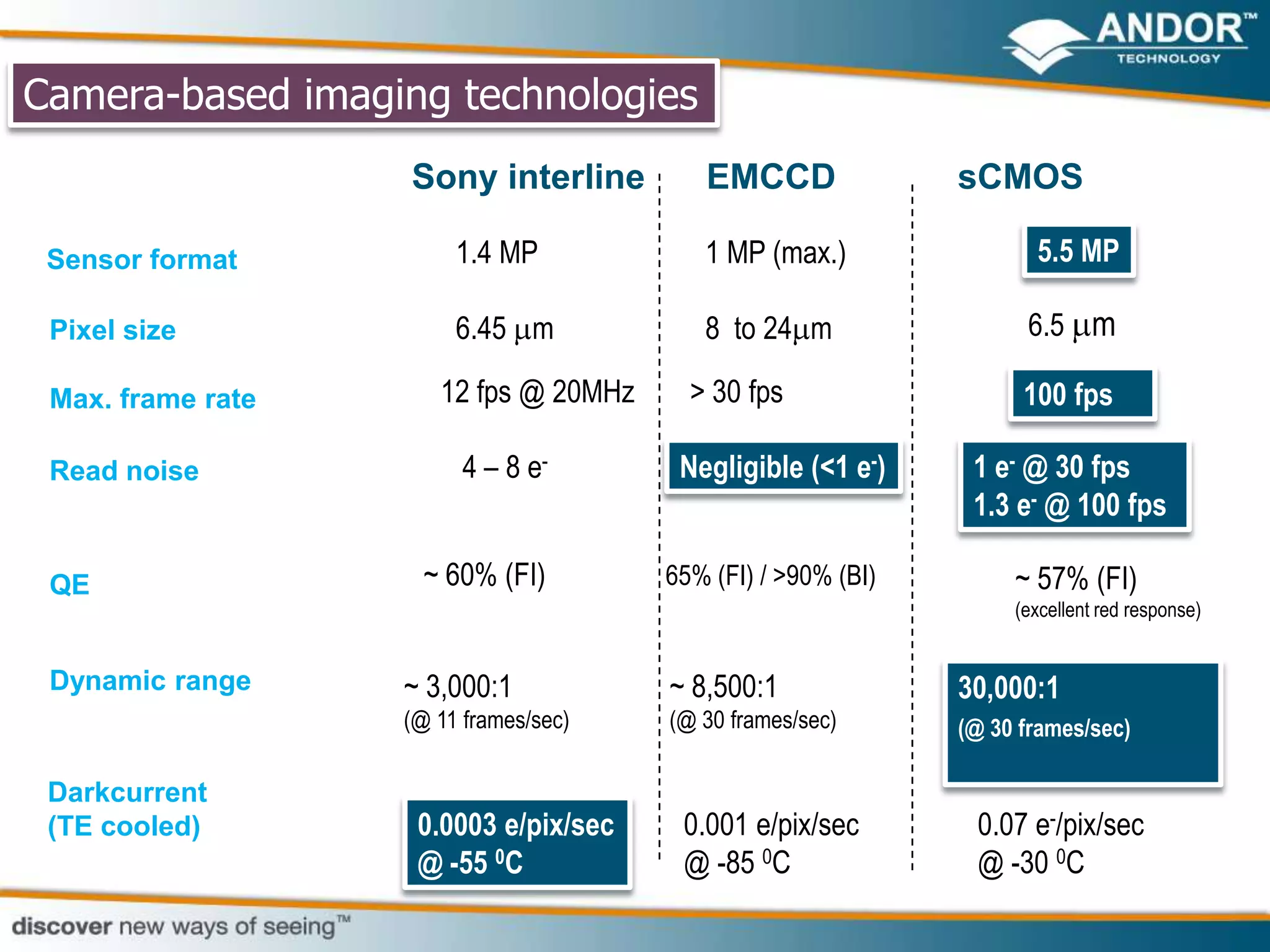
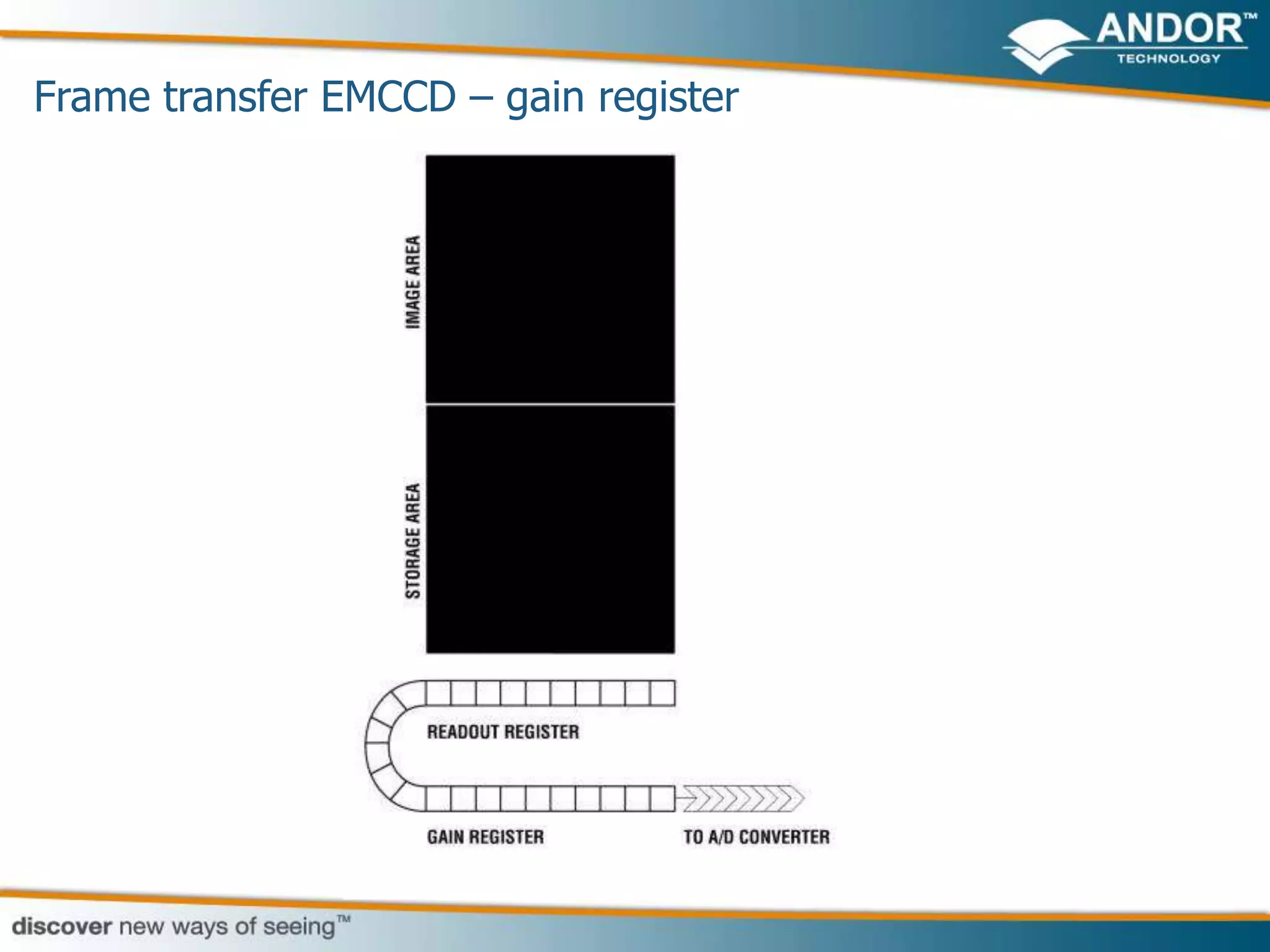
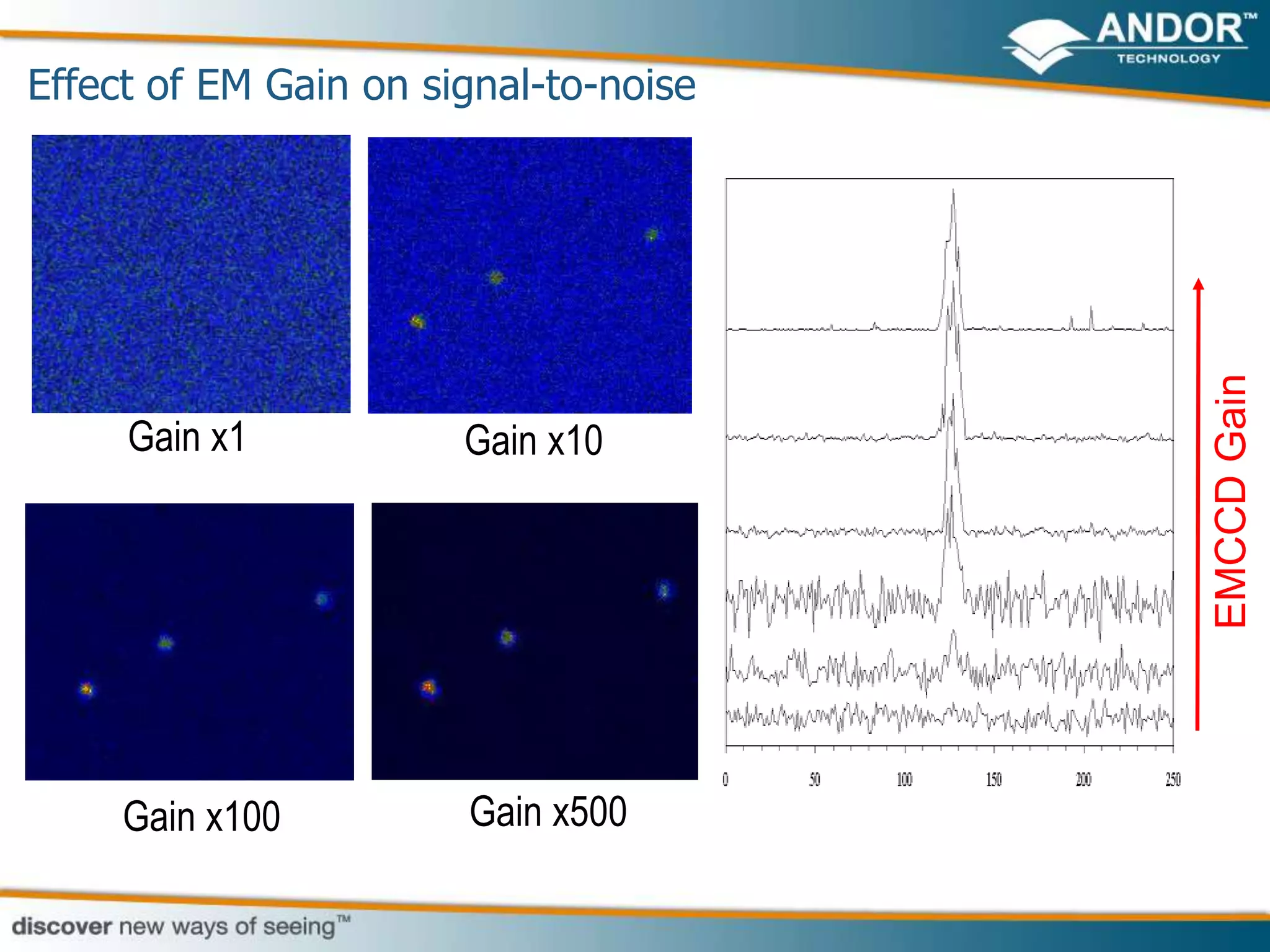
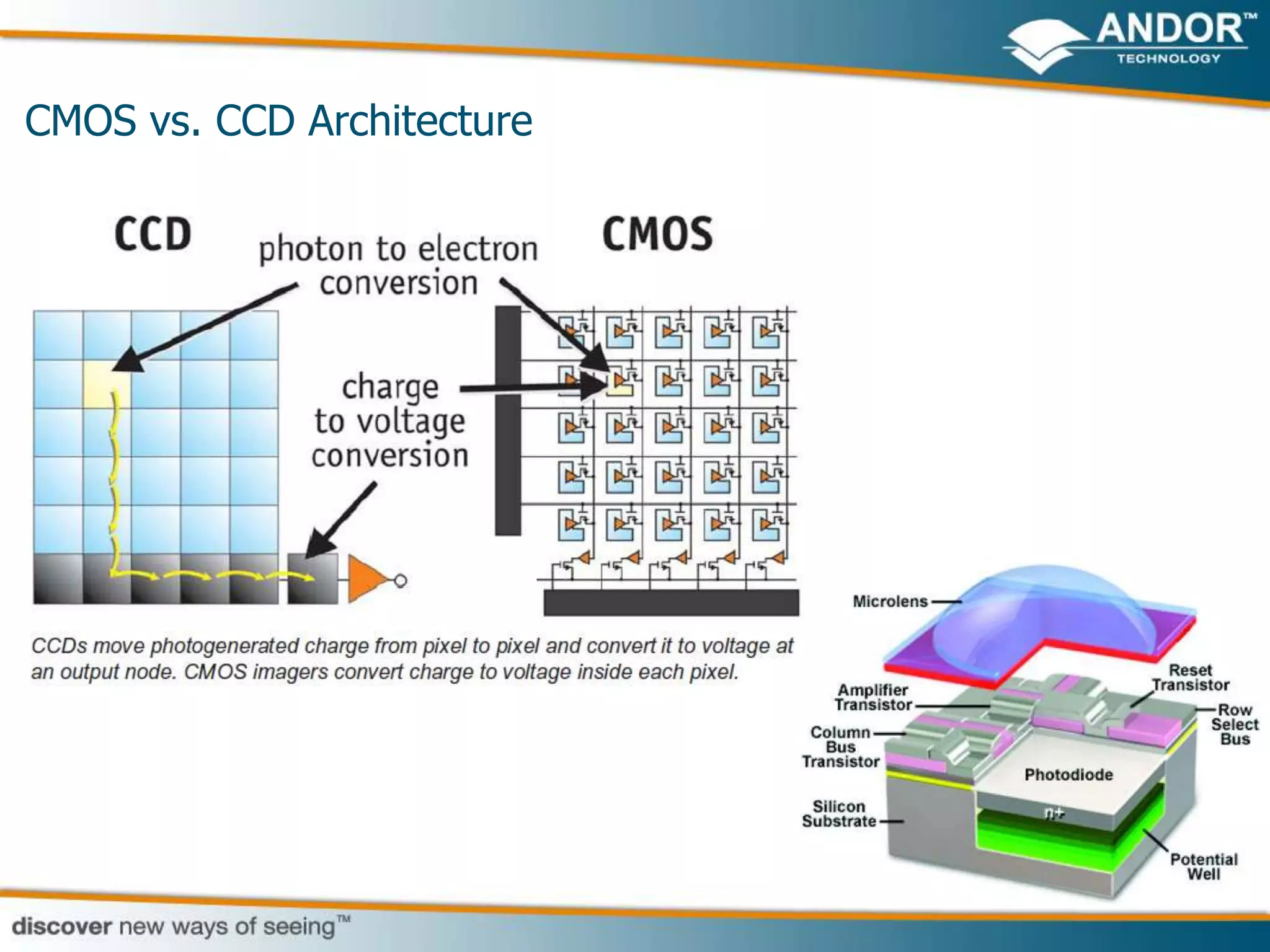
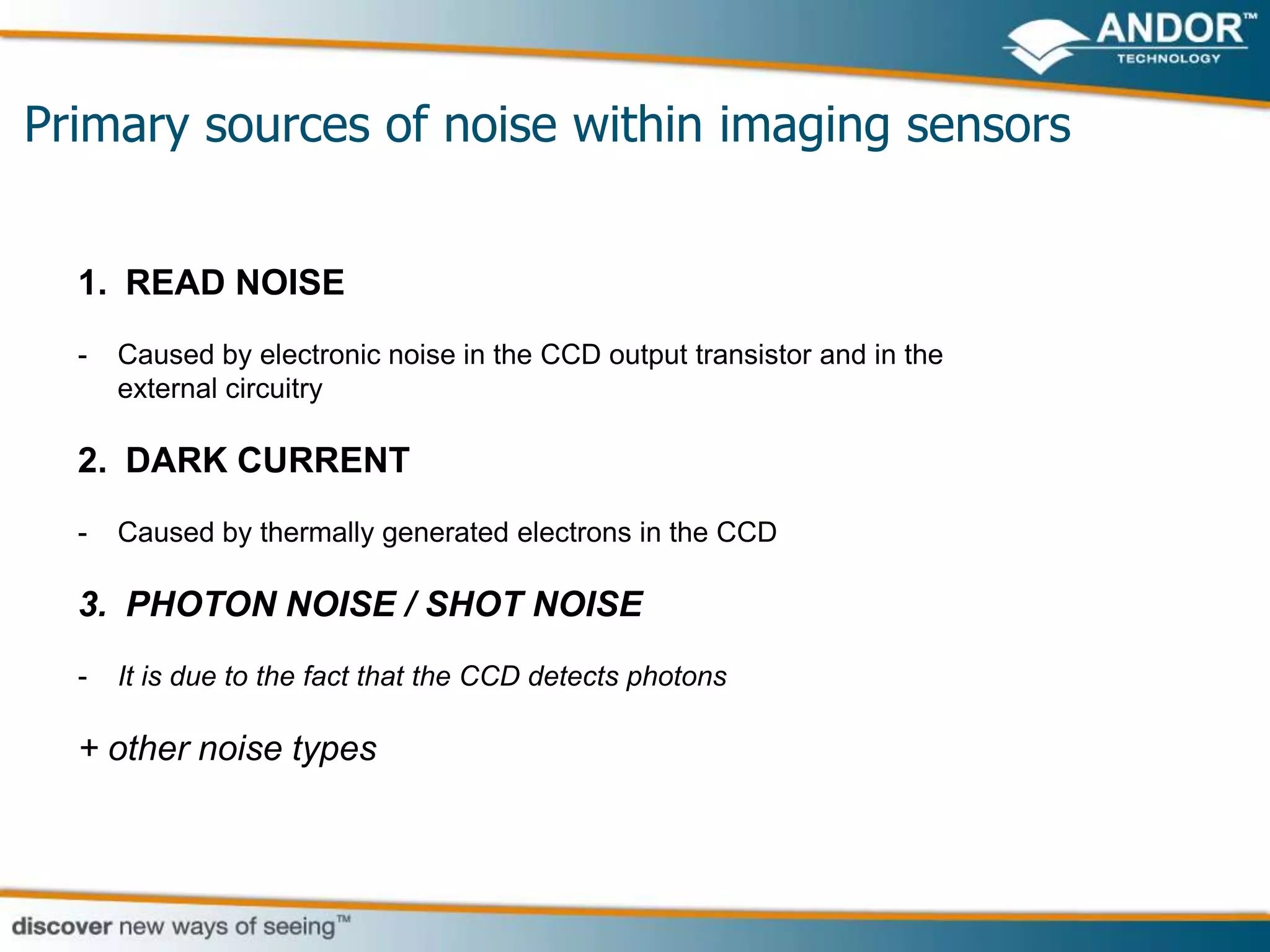
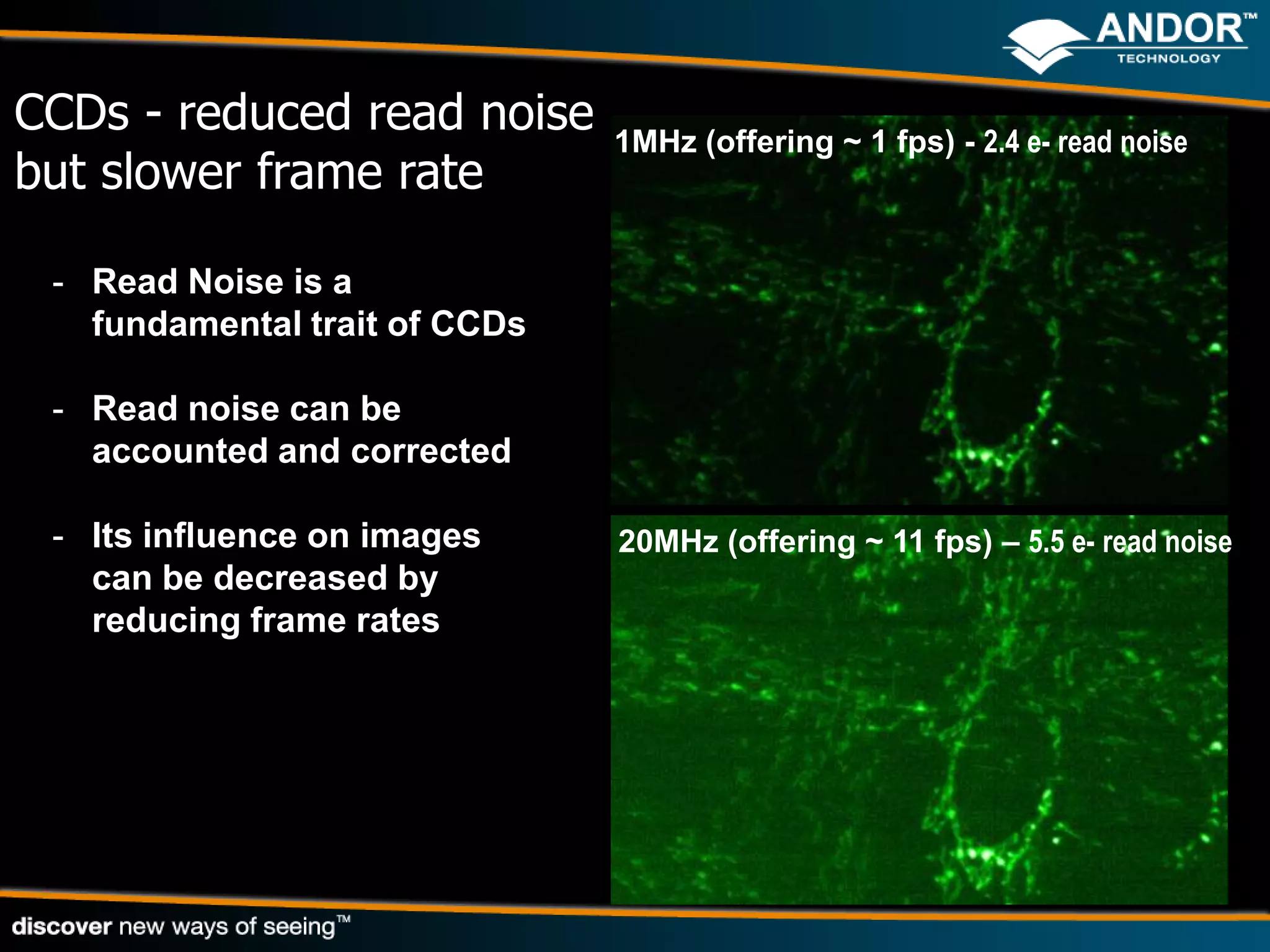
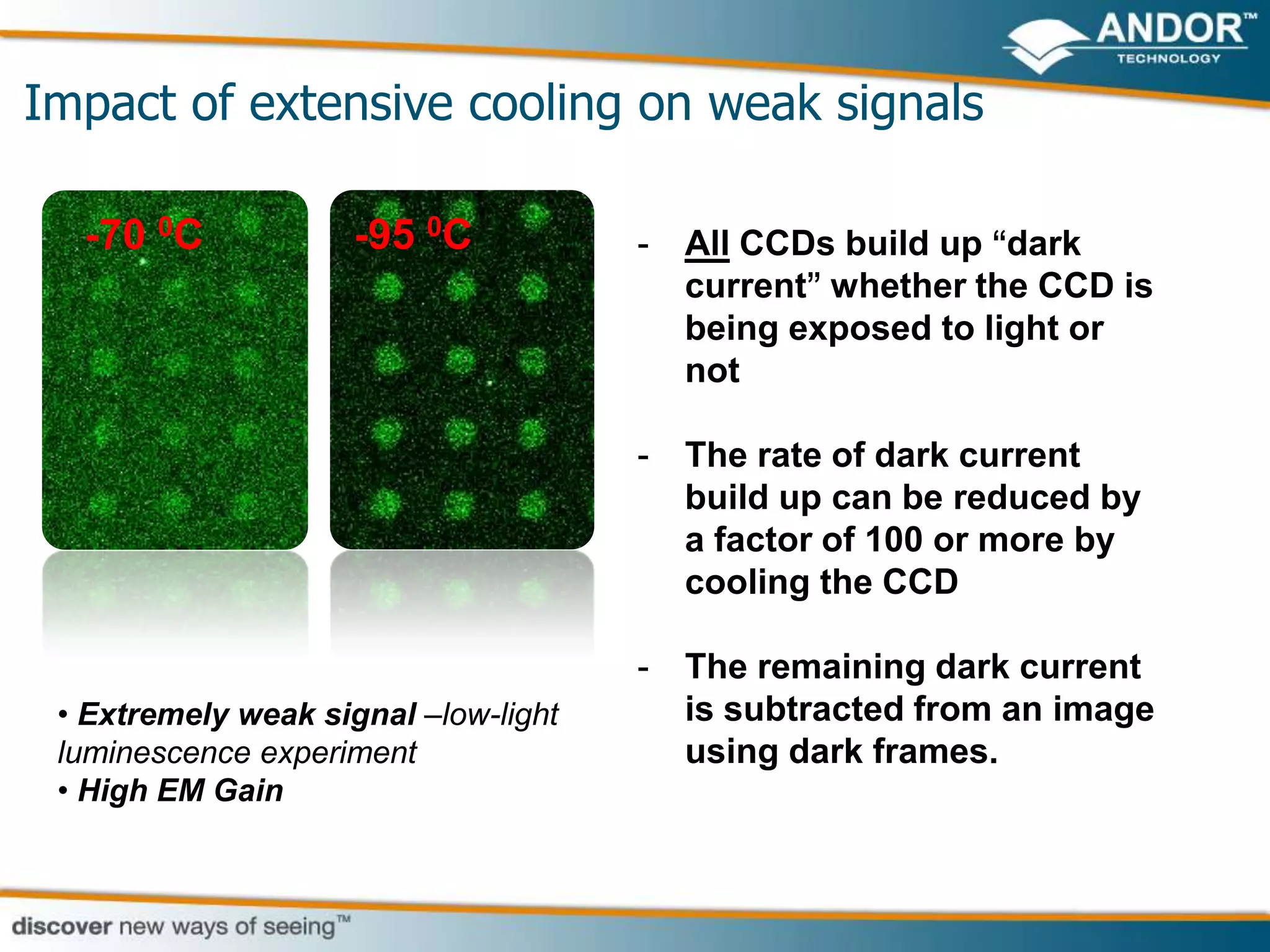
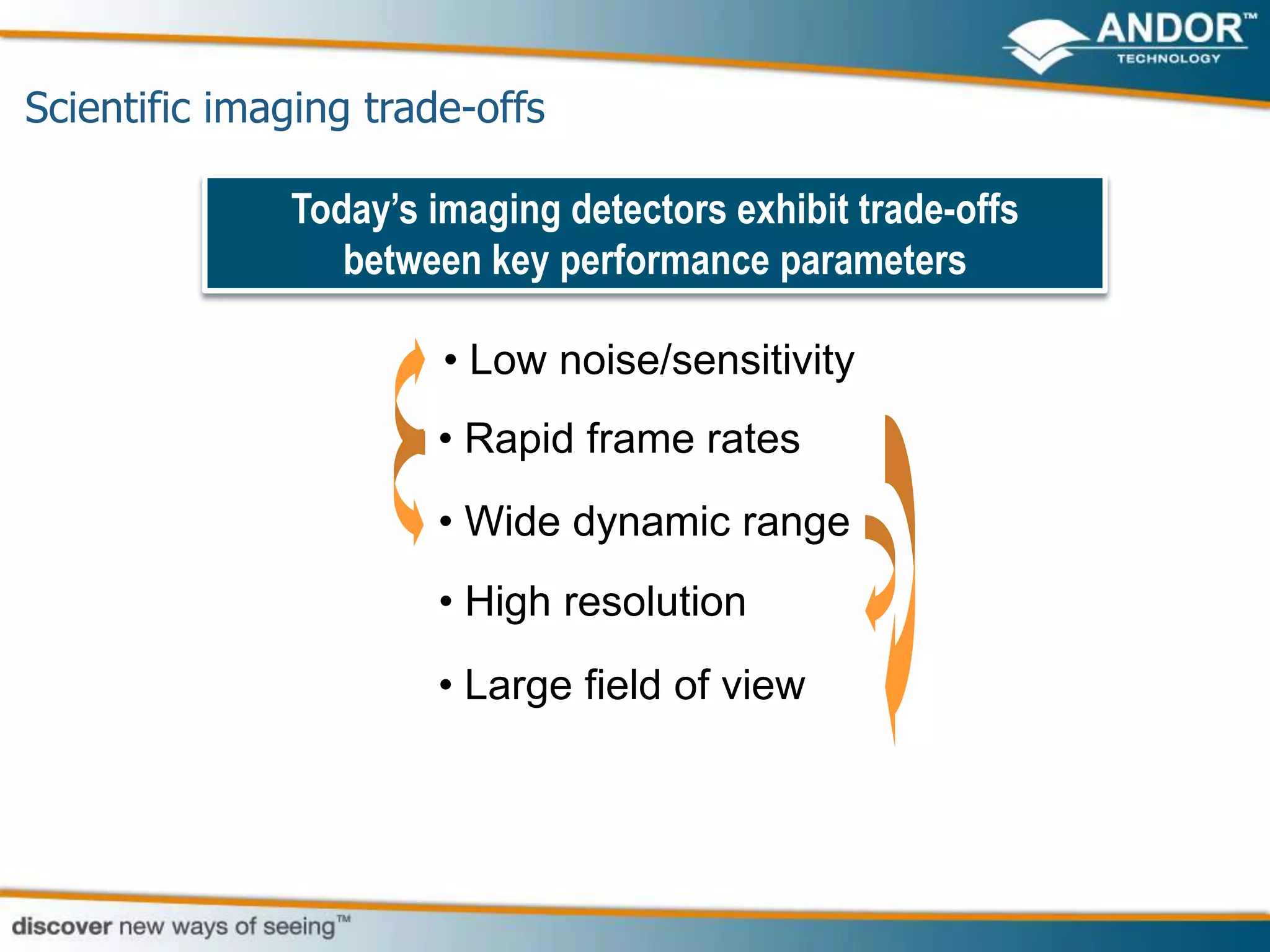
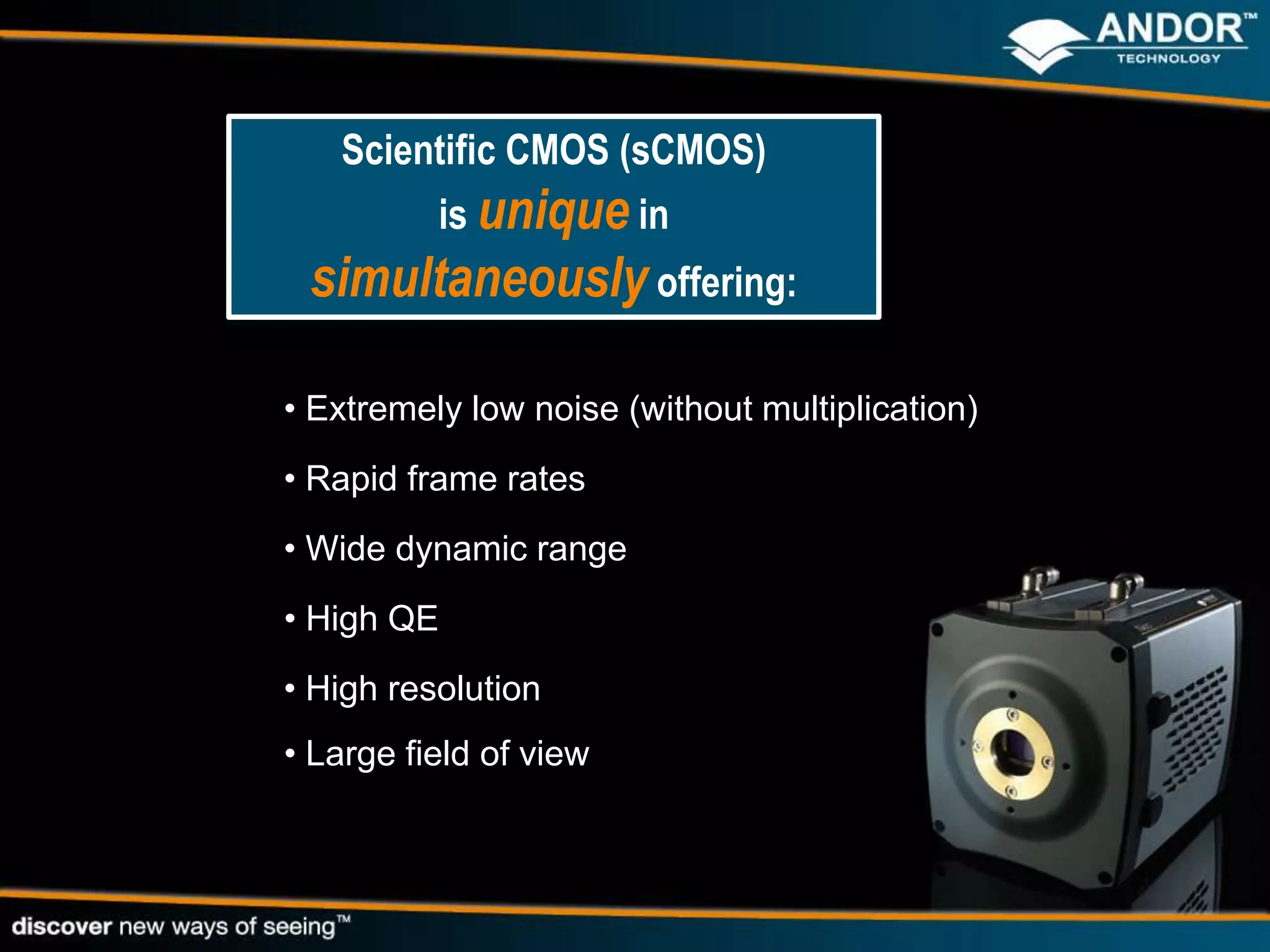
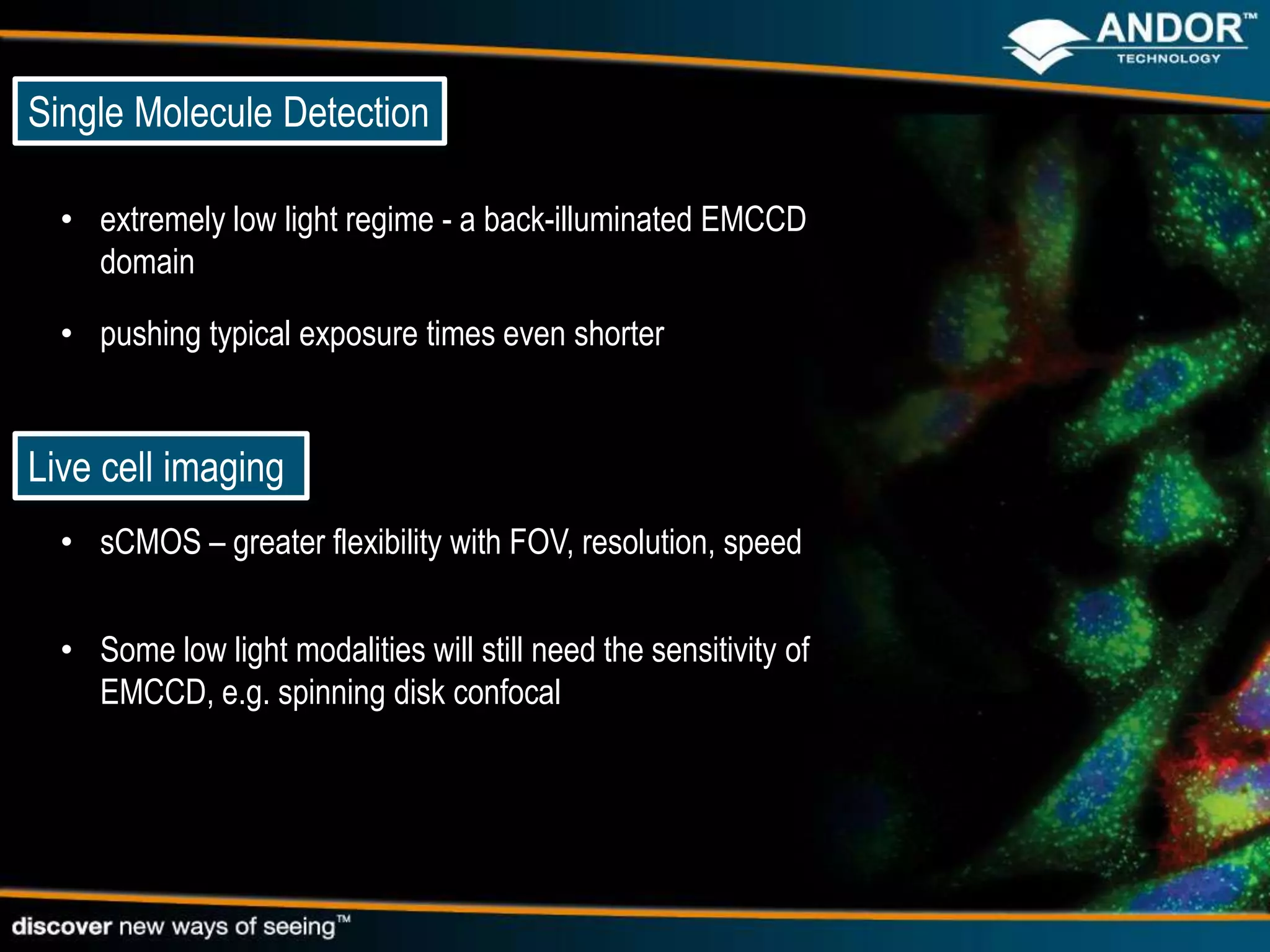
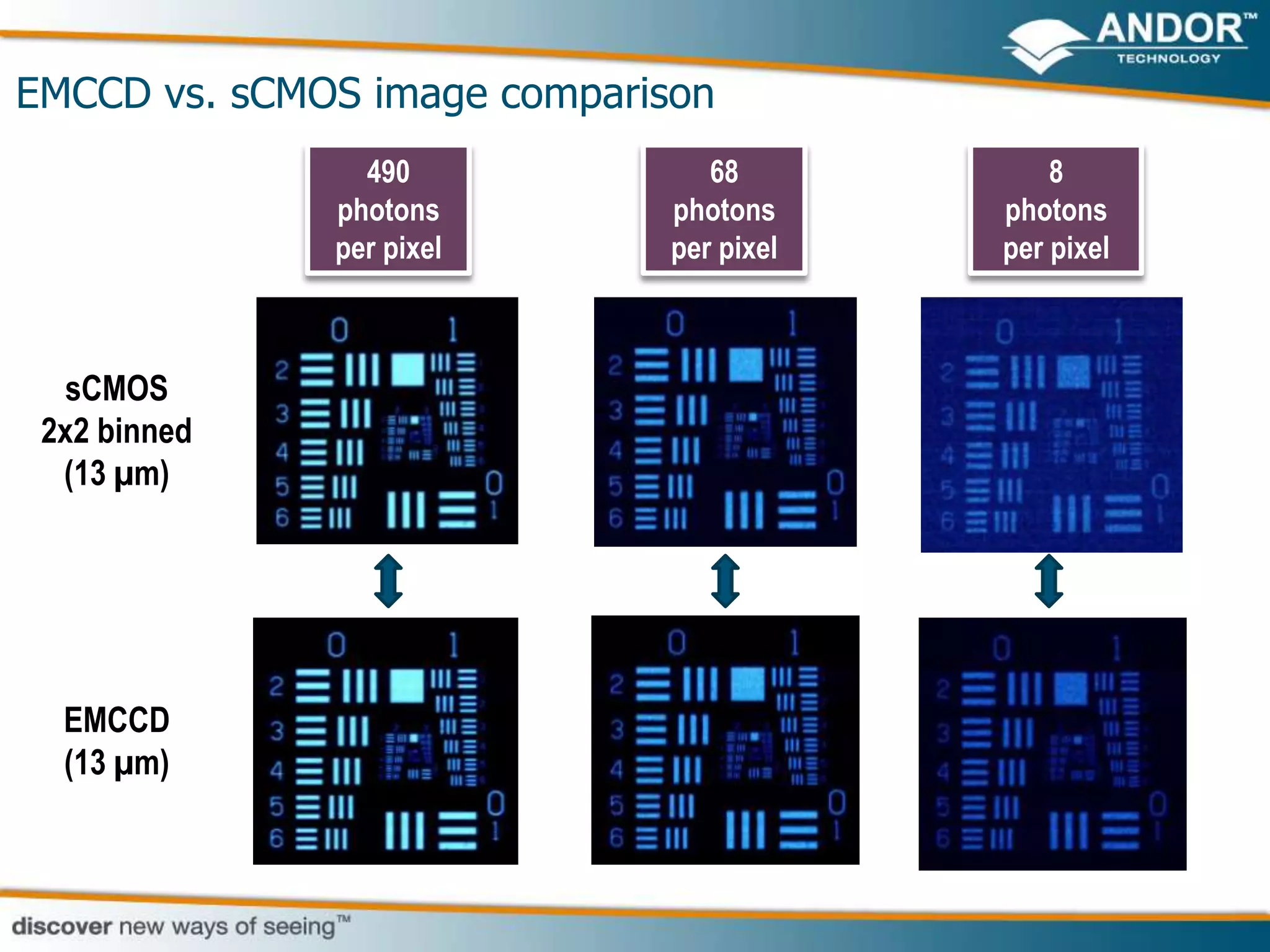
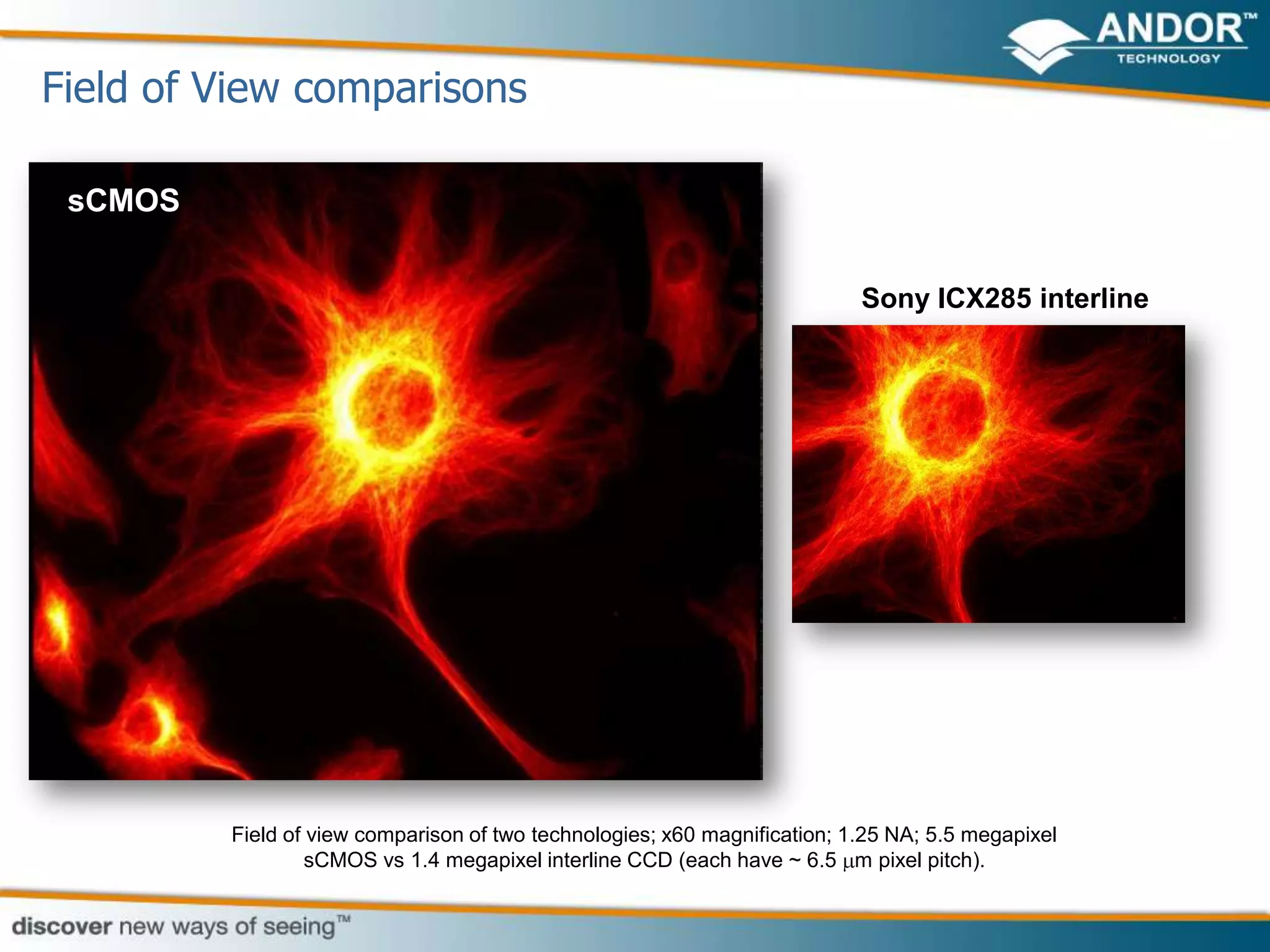
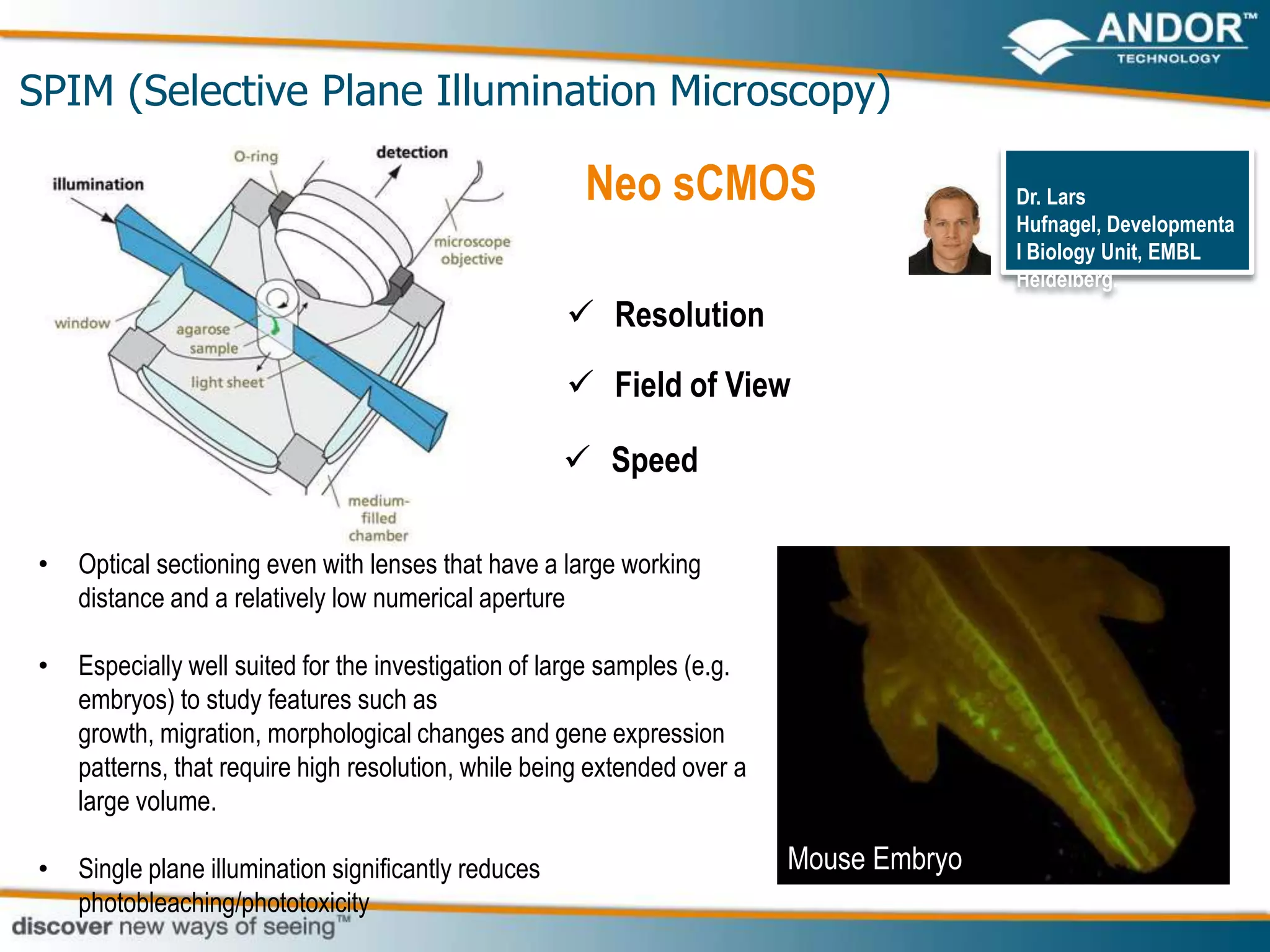
This document discusses light microscopy detectors. It compares different types of detectors including PMTs, APDs, CCDs, and CMOS, noting their strengths and weaknesses in terms of speed, noise levels, resolution, and other factors. It focuses on how detectors can be optimized for sensitivity, discussing parameters like quantum efficiency and noise floor. Specific detector technologies are examined in more detail, such as EMCCDs and scientific CMOS cameras, comparing their performance and applications in areas like single molecule detection and live cell imaging.