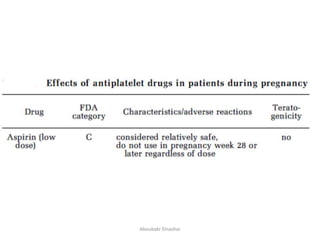
This document discusses the use of low dose aspirin in obstetrics. It covers the safety, mechanism of action, and various uses of LDA including: prevention of preeclampsia, IUGR, preterm labor, hypertension in multiple pregnancies, and recurrent miscarriage. It finds that LDA is generally safe for mothers and fetuses. It effectively prevents preeclampsia and other adverse outcomes in high-risk pregnancies without major safety risks. The mechanism of action involves diminishing platelet thromboxane A2 synthesis while maintaining prostacyclin synthesis. Guidelines recommend LDA for women with certain risk factors starting between 12-16 weeks.




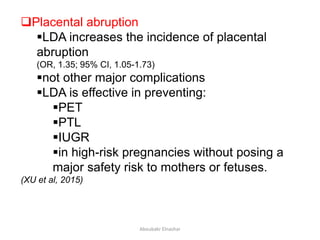




![I. Studies on moderate and high risk women
low dose aspirin: effective
modest reduction in risk of
PE (0-3% in treated vs 12-35% in controls)
other adverse pregnancy outcome:
PTL, IUGR (by 10-20%).
[Dekker et al, 2001].level 2 evidence (Cochrane SR, 2007 )
Aboubakr Elnashar](https://image.slidesharecdn.com/aspirinpreg-161223224714/85/low-dose-Aspirin-in-obstetrics-11-320.jpg)

![Dose:
75-80 mg seems to be the right dosage for about
two thirds of the women
[Rey et al, 2011]
1/3: need higher dosages up to 160 mg
Aspirin resistance test:
woman is resistant to 75-80 mg: increase the
dose.
(Bujold, 2013)
100 mg/d should be the minimum dose for
prevention of complications in pregnancy
(Ayal et al, 2013)
Aboubakr Elnashar](https://image.slidesharecdn.com/aspirinpreg-161223224714/85/low-dose-Aspirin-in-obstetrics-13-320.jpg)



![II. Unselected nulliparous women
little or no benefit
[Sibai et al, 1993]
no effect on incidence of FGR, or length of
gestation
[Subtil et al, 2003].
1. Although nulliparity is a risk factor for PE,
prevalence rates are relatively low (4%)
compared with moderate to high risk groups
(8-30%)
[Henderson et al, 2014].
2. Pathogenesis of PE in nulliparous is
different from that in women with previous
PE or preexisting vascular disease
[Sibai et al, 2005].
Aboubakr Elnashar](https://image.slidesharecdn.com/aspirinpreg-161223224714/85/low-dose-Aspirin-in-obstetrics-17-320.jpg)
![III. Studies on women with abnormal uterine artery
Doppler (UAD)
Abnormal UAD: identified women who are likely
to develop PE and IUGR
[Subtil et al, 2003].
PE: 6 vs 1%
IUGR: 18 vs 8%
LDA of abnormal UAD:
Did not reduce the incidence of PE
PE occurred in 2% of patients in each group.
Did not reduce the incidence of IUGR.
Aboubakr Elnashar](https://image.slidesharecdn.com/aspirinpreg-161223224714/85/low-dose-Aspirin-in-obstetrics-18-320.jpg)














