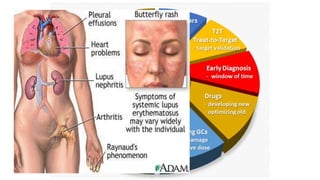
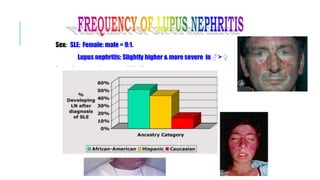
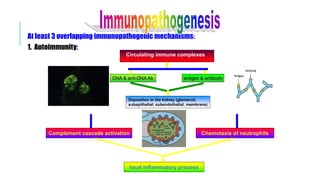
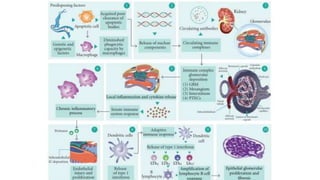
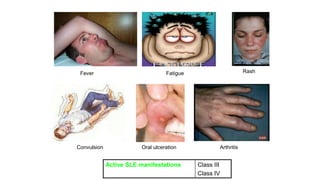
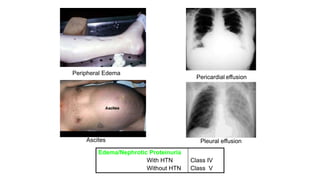
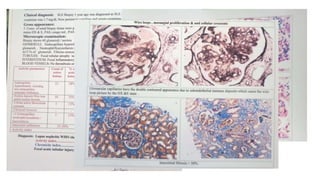
- The document discusses lupus nephritis, its incidence, presentation, pathogenesis, classification, and management.
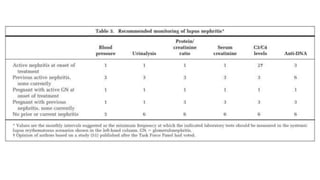
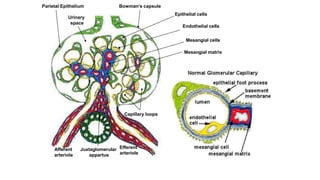
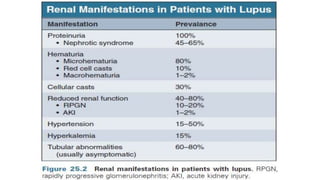
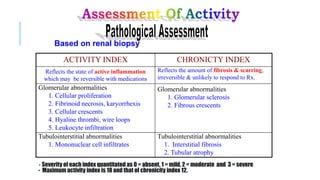
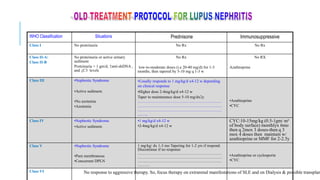
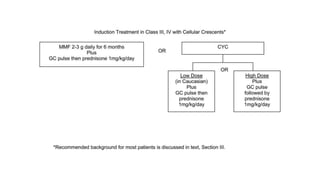
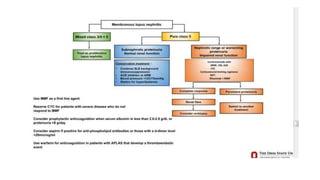
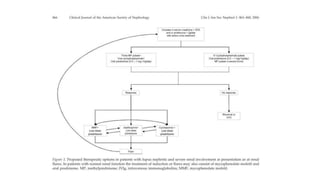
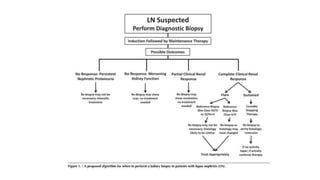
- Renal biopsy is important for assessing disease activity and chronicity to guide treatment, which typically involves immunosuppressants like mycophenolate or cyclophosphamide combined with corticosteroids.
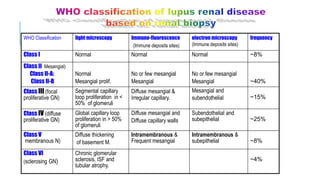
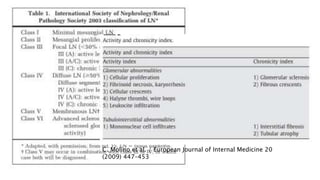
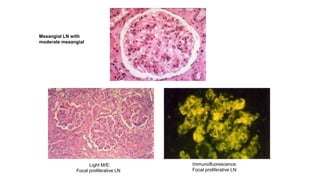
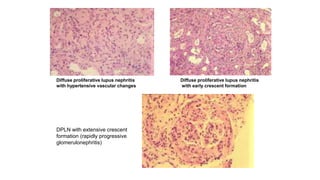
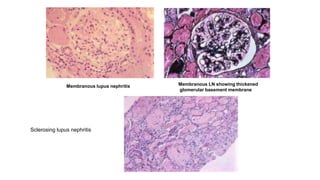
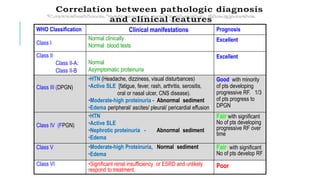
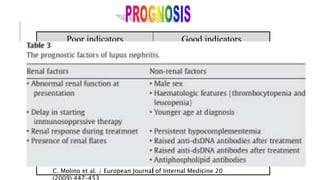
- Prognosis depends on the WHO classification - classes I-II have excellent prognosis while classes IV-VI have poorer renal outcomes if not treated aggressively.







![↑ESR
↑ anti-dsDNA, anti-Sm Ab especially with FPLN and DPLN
↓ C3 & C4 levels
↓Total hemolytic complement (CH50)
↓Serum albumin and ↑serum cholesterol.
↑ Serum creatinine (in renal dysfunction]
30% ↓ creatinine clearance (in 40-80% of pts).
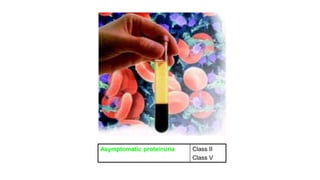
↑Proteinuria >1000 mg/d ± active sediment (leukocyturia, hematuria, and
granular & hyaline casts.](https://image.slidesharecdn.com/slecomplication-210930172220/85/Sle-complication-24-320.jpg)









![Other therapies:
Plasmapheresis-
Remove immune complexes & autoantibodies
Most useful in lupus pts w/ thrombotic microangiopathic
hemolytic anemia or 2ry TTP.
2-4 L (40mg/kg] of plasma are removed & replaced with albumin-
saline solution ± 1-2 units of fresh frozen plasma.
Most useful if given in combination with corticosteroids &
cytotoxic drugs.](https://image.slidesharecdn.com/slecomplication-210930172220/85/Sle-complication-42-320.jpg)


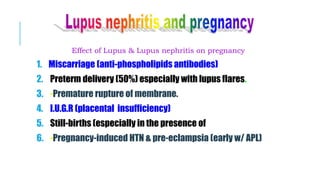
![Effect of Lupus & Lupus nephritis on baby
Risk of neonatal lupus in 5% of the new-born induced by mother’s
antibodies ( anti-Ro & anti-La) that cross the placenta to the fetus.
Neonatal lupus is characterized by rashes which disappear in 3-6
mo. [not lupus & not turn to lupus].
Some babies have congenital heart block, irreversible CHF and
blood abnormalities (pancytopenia).](https://image.slidesharecdn.com/slecomplication-210930172220/85/Sle-complication-50-320.jpg)

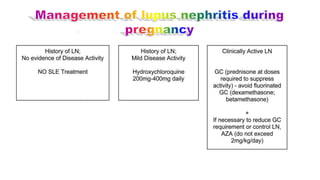
![Treatment
Flares of lupus nephritis during pregnancy should be treated
with steroids and azathiaprine. Steroids, azathiaprine,
methyldopa and diuretics are safe in pregnancy.
Continuation of immunosuppressive treatment for at least 2
months after delivery is advised. (Postpartum flares may be
related to high levels of prolactin in lactating mothers ].
Very severe exacerbation of lupus in pregnancy may require
termination of pregnancy but the disease will not necessarily
improve.](https://image.slidesharecdn.com/slecomplication-210930172220/85/Sle-complication-54-320.jpg)