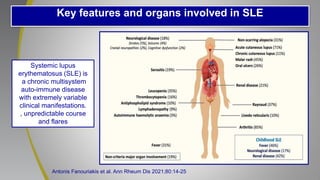
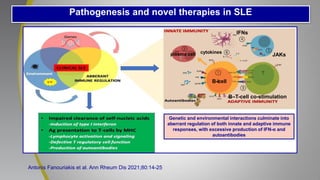
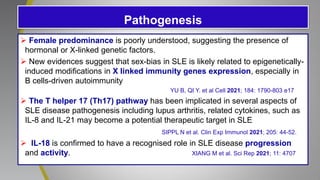
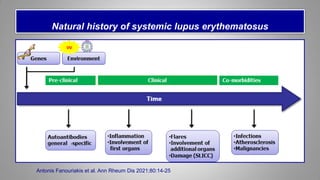
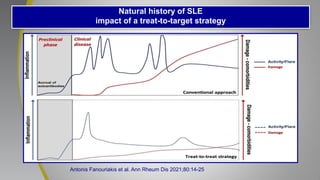
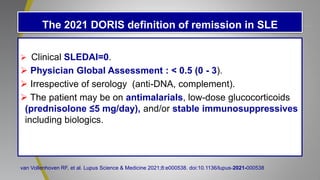
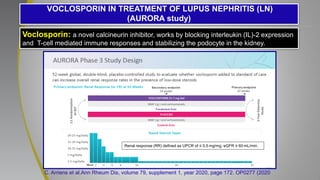
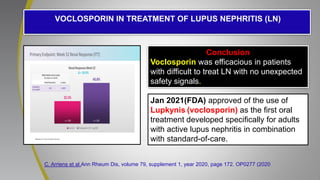
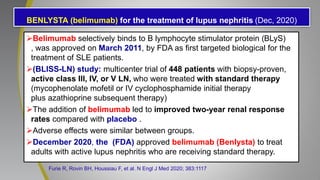
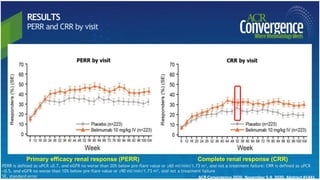
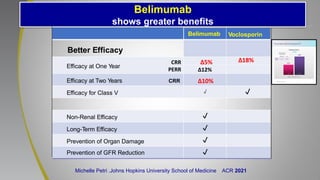
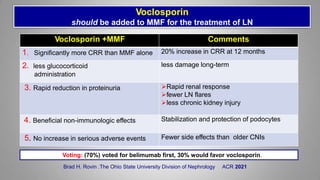
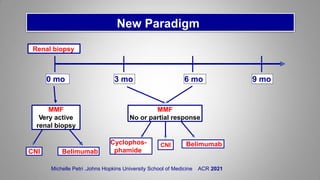
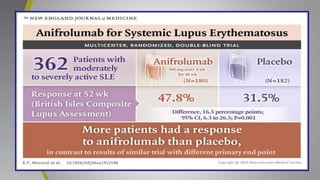
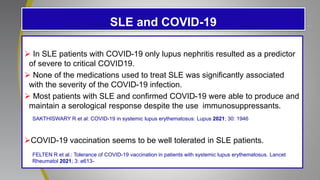
The document provides an update on systemic lupus erythematosus (SLE), covering recent findings in its pathogenesis, treatment strategies, and the emergence of new therapies. It highlights the importance of a treat-to-target approach and the 2021 definition of remission, as well as introducing new medications like voclosporin and belimumab, which have shown efficacy in lupus nephritis. Additionally, it discusses the impact of COVID-19 on SLE patients, emphasizing that lupus nephritis is a predictor for severe COVID-19 outcomes.