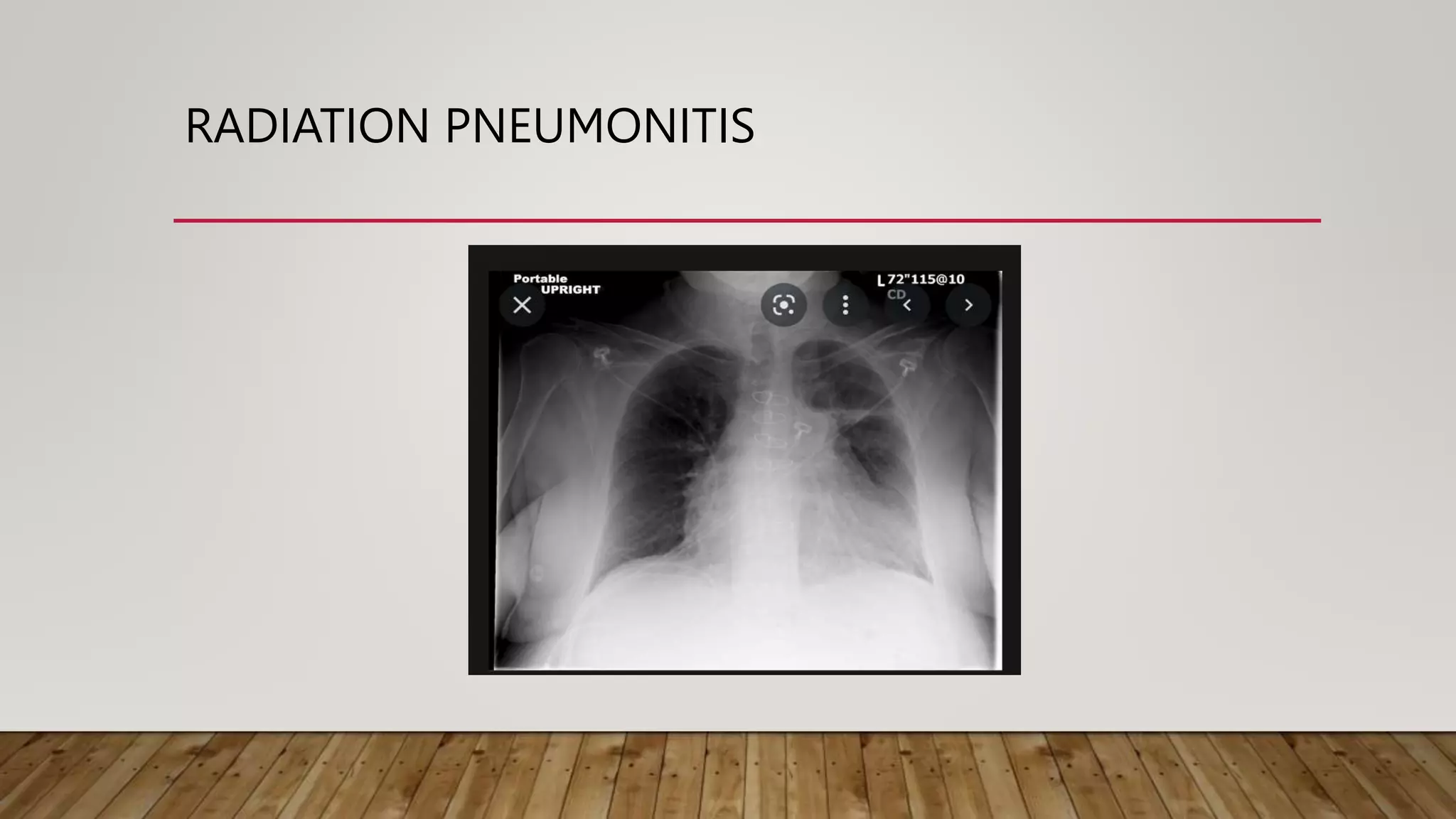
This document discusses the late effects of radiotherapy. It notes that while radiotherapy effectively treats cancer by damaging DNA in cancer cells, it can also affect normal cells, leading to both acute and late effects. Late effects occur more than 3 months after treatment and involve tissues with slow turnover rates. Common late effects include fibrosis, atrophy, necrosis, vascular damage, and secondary malignancies. The document outlines late effects for various organs and tissues. It emphasizes the importance of prevention strategies like treatment planning and education to minimize complications and improve patient outcomes and quality of life.