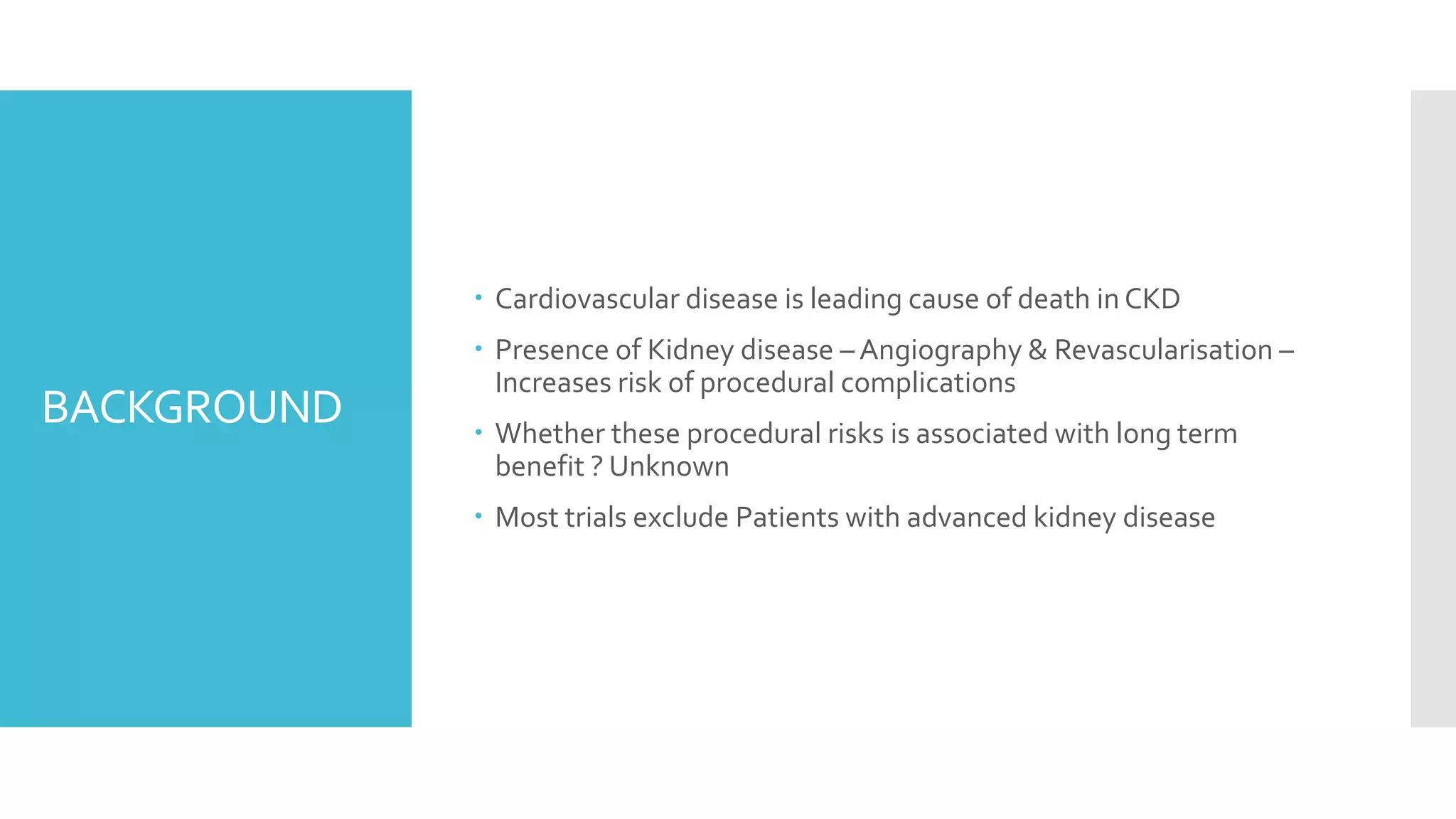
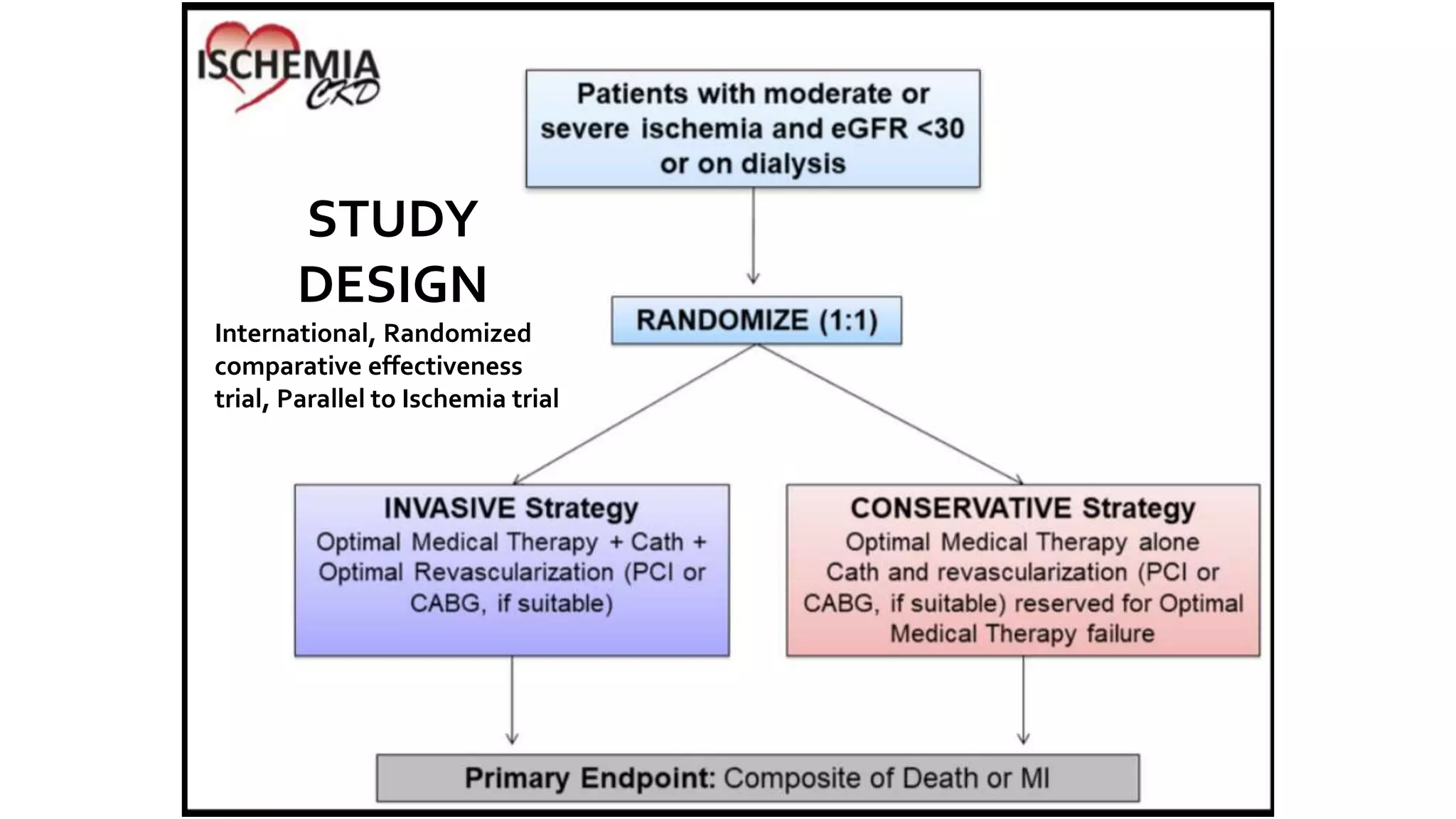
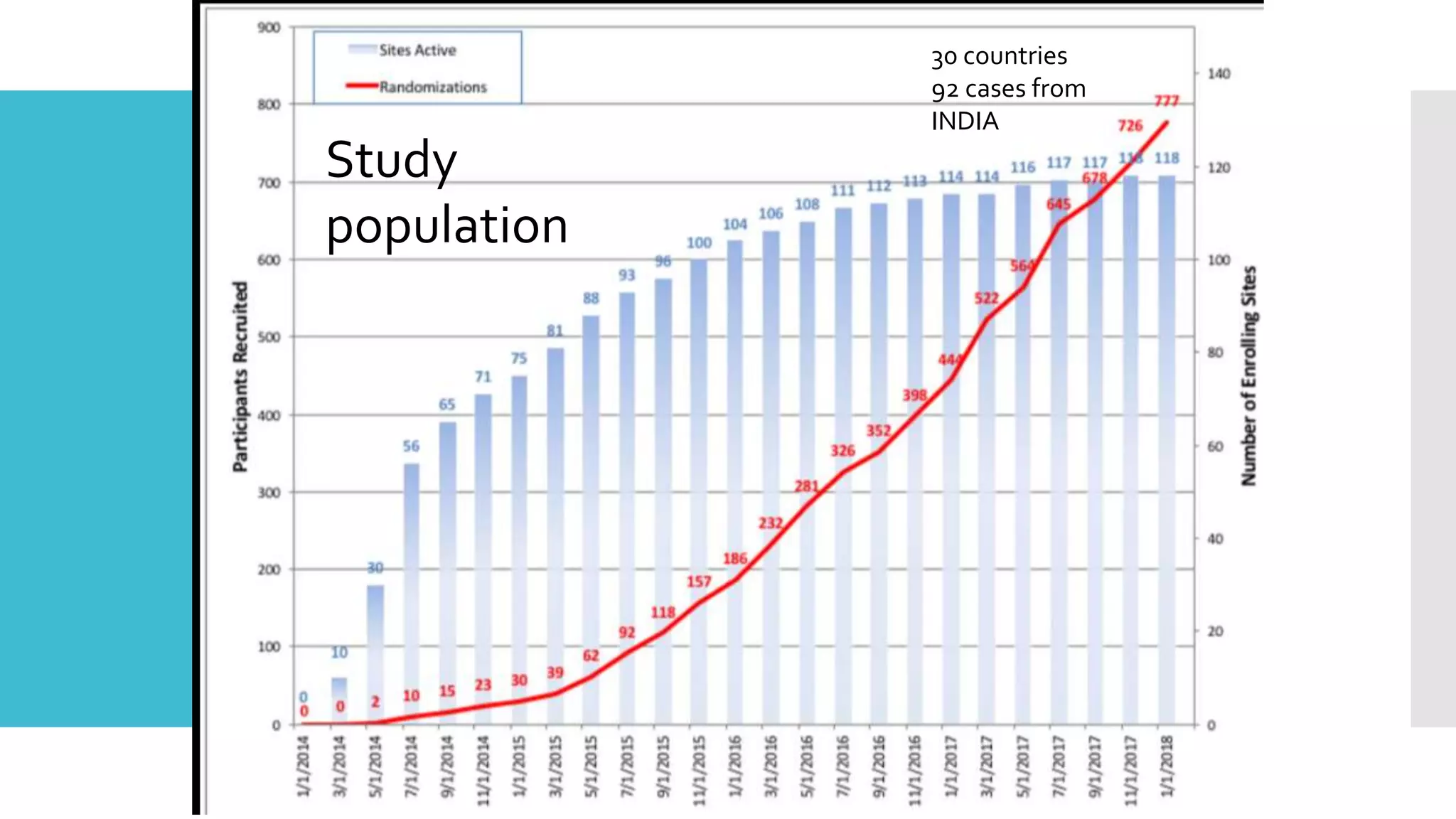
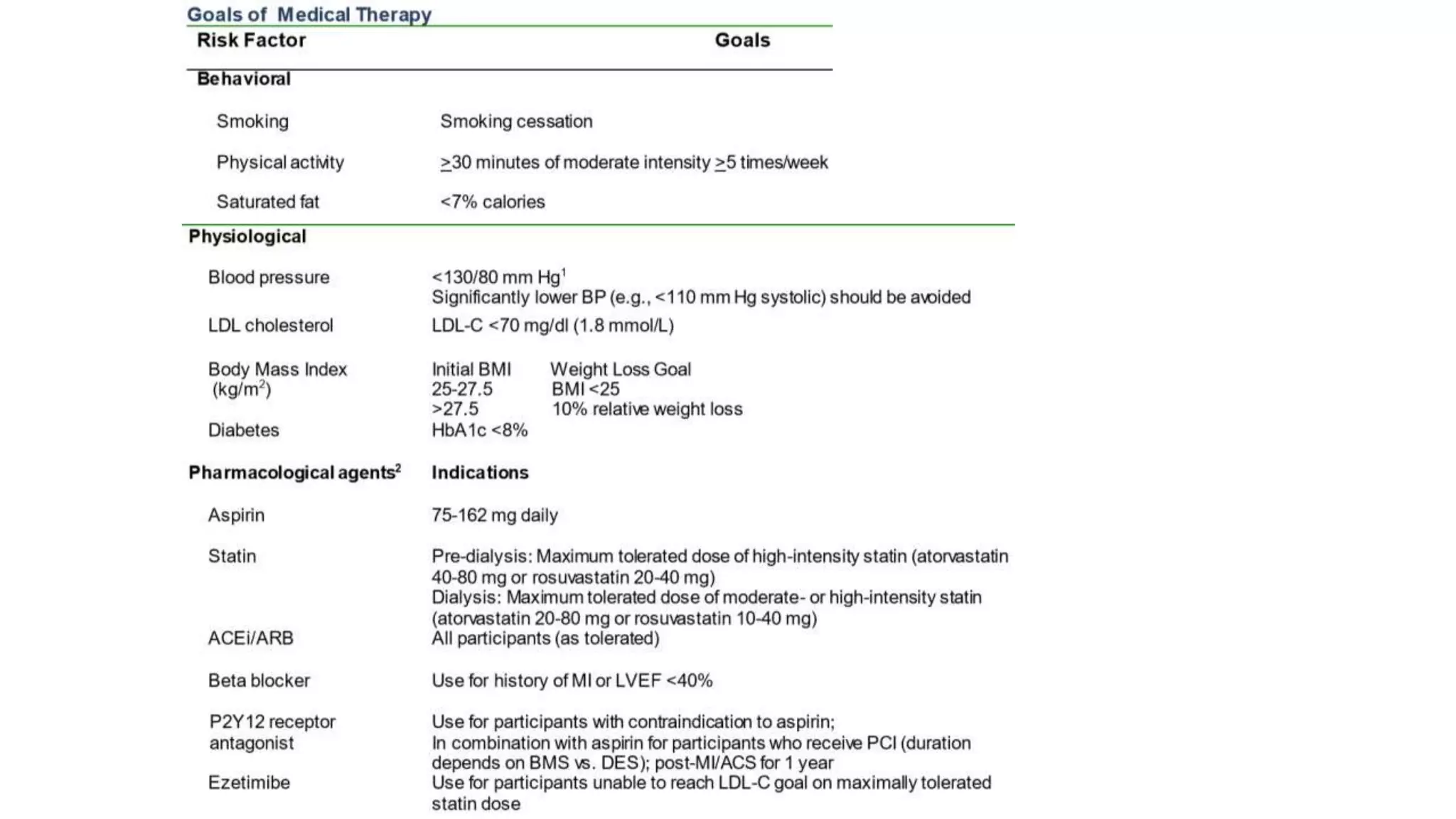
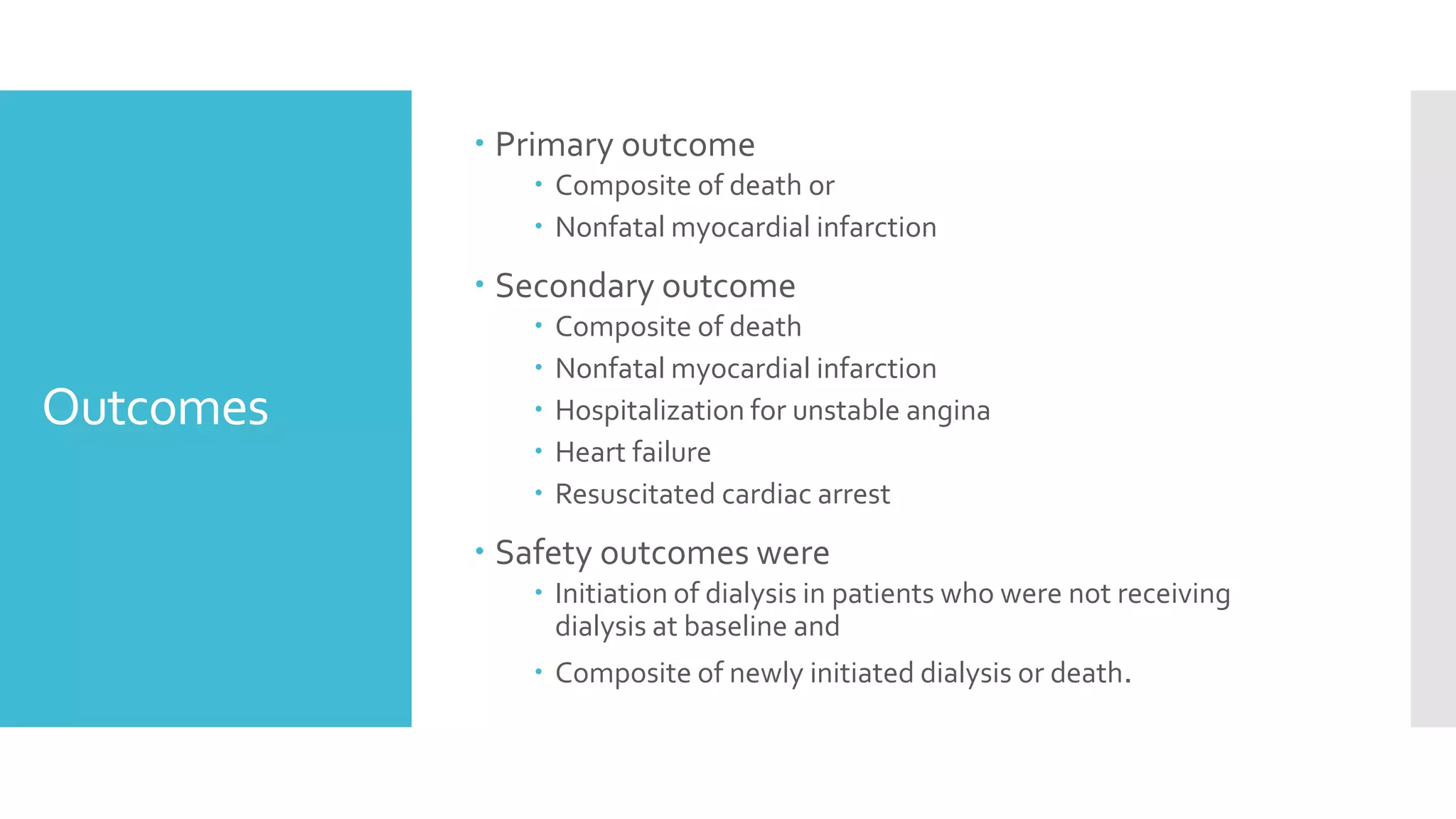
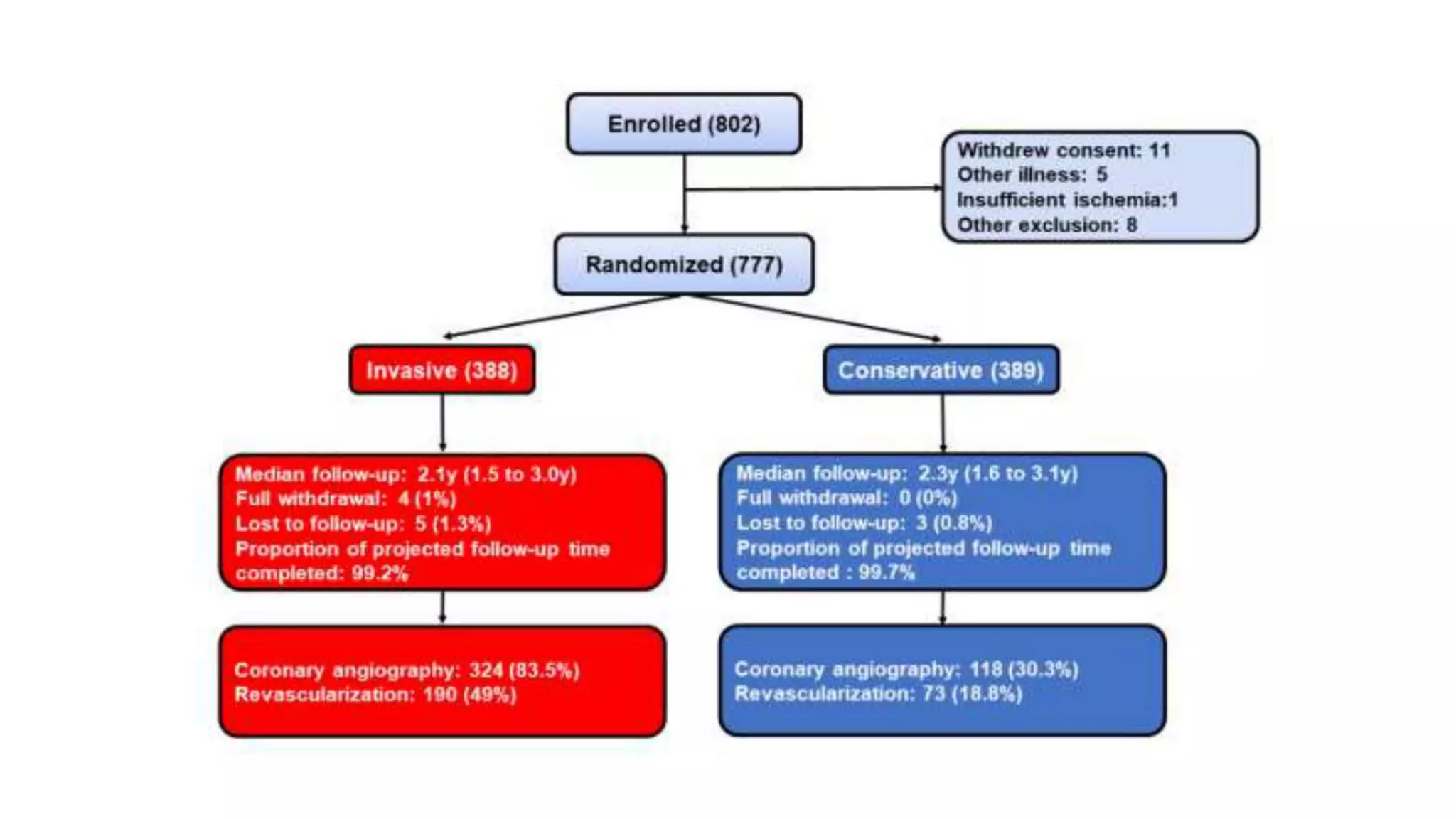
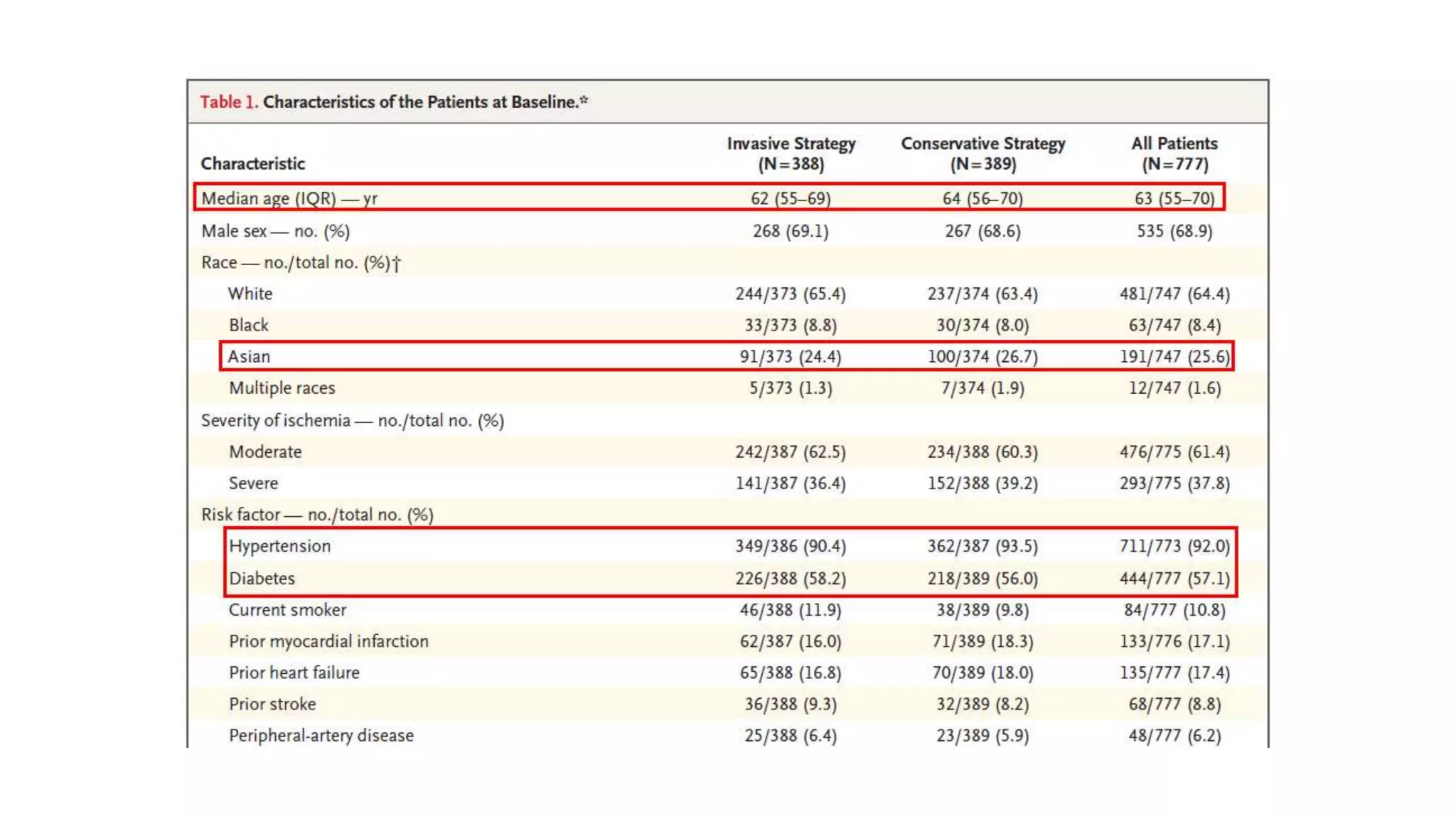
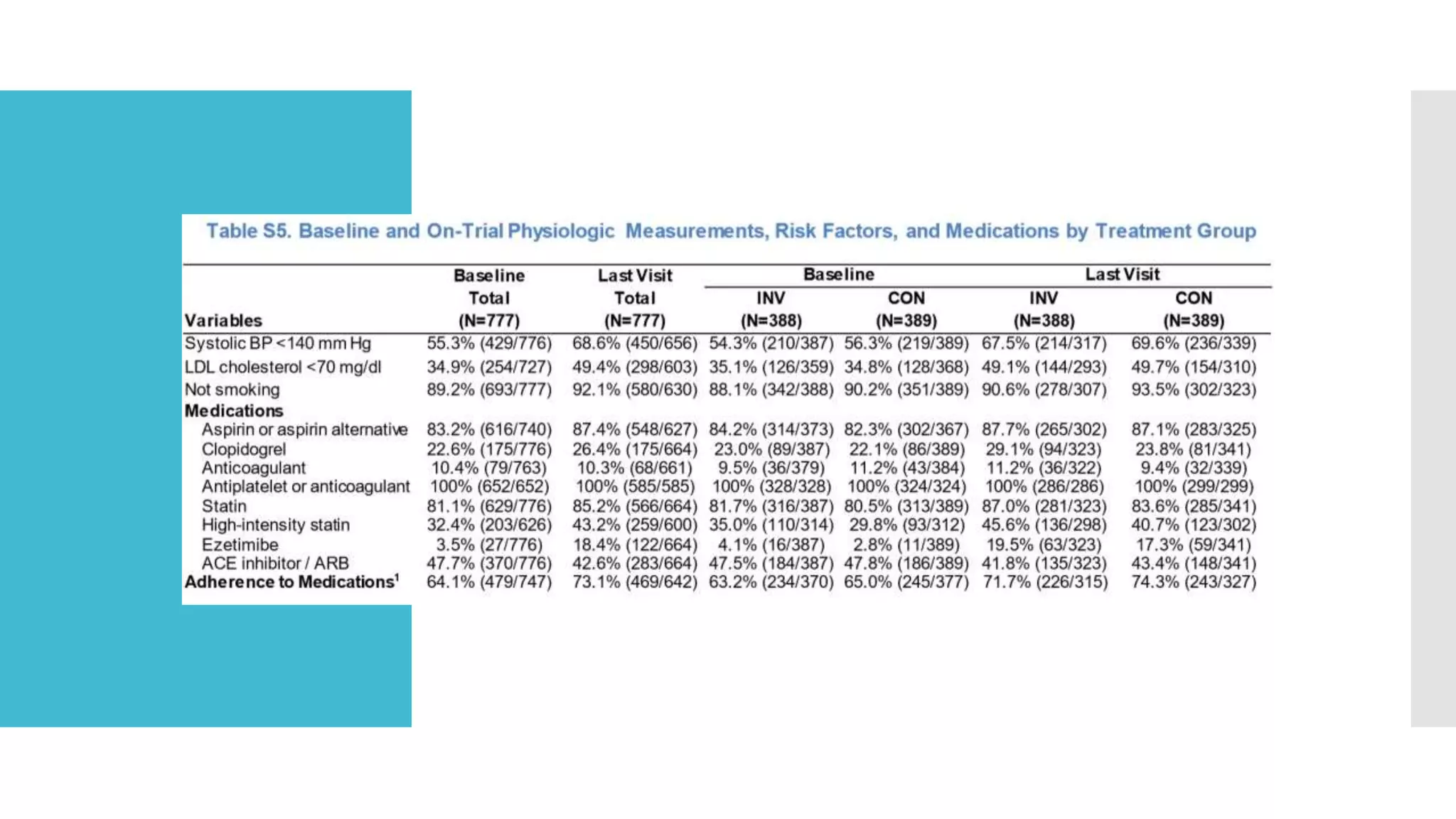
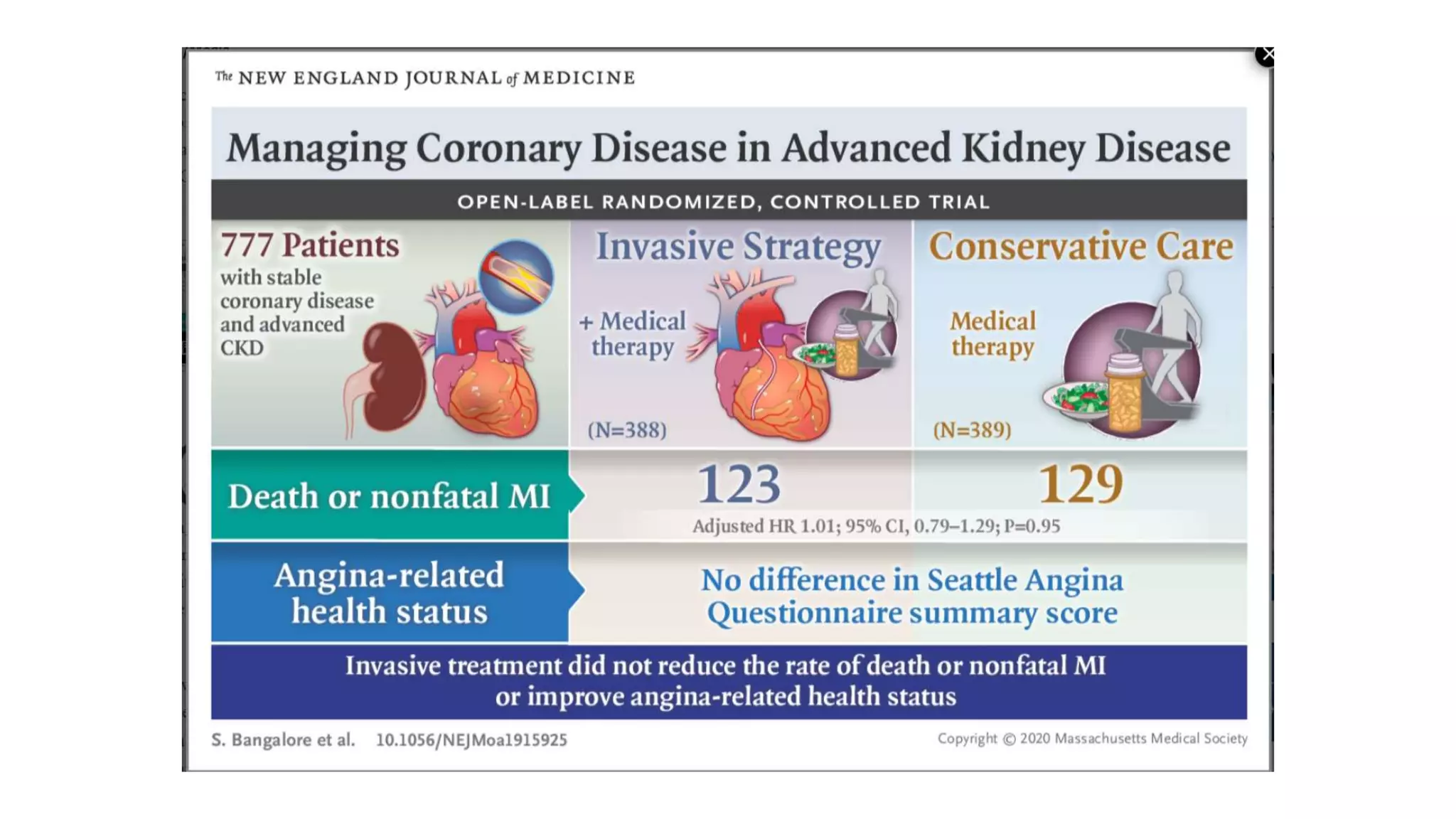
1) The document describes a randomized controlled trial called ISCHEMIA-CKD that compared an invasive strategy (PCI or CABG) to a conservative strategy in patients with stable coronary disease, advanced chronic kidney disease, and moderate or severe ischemia.
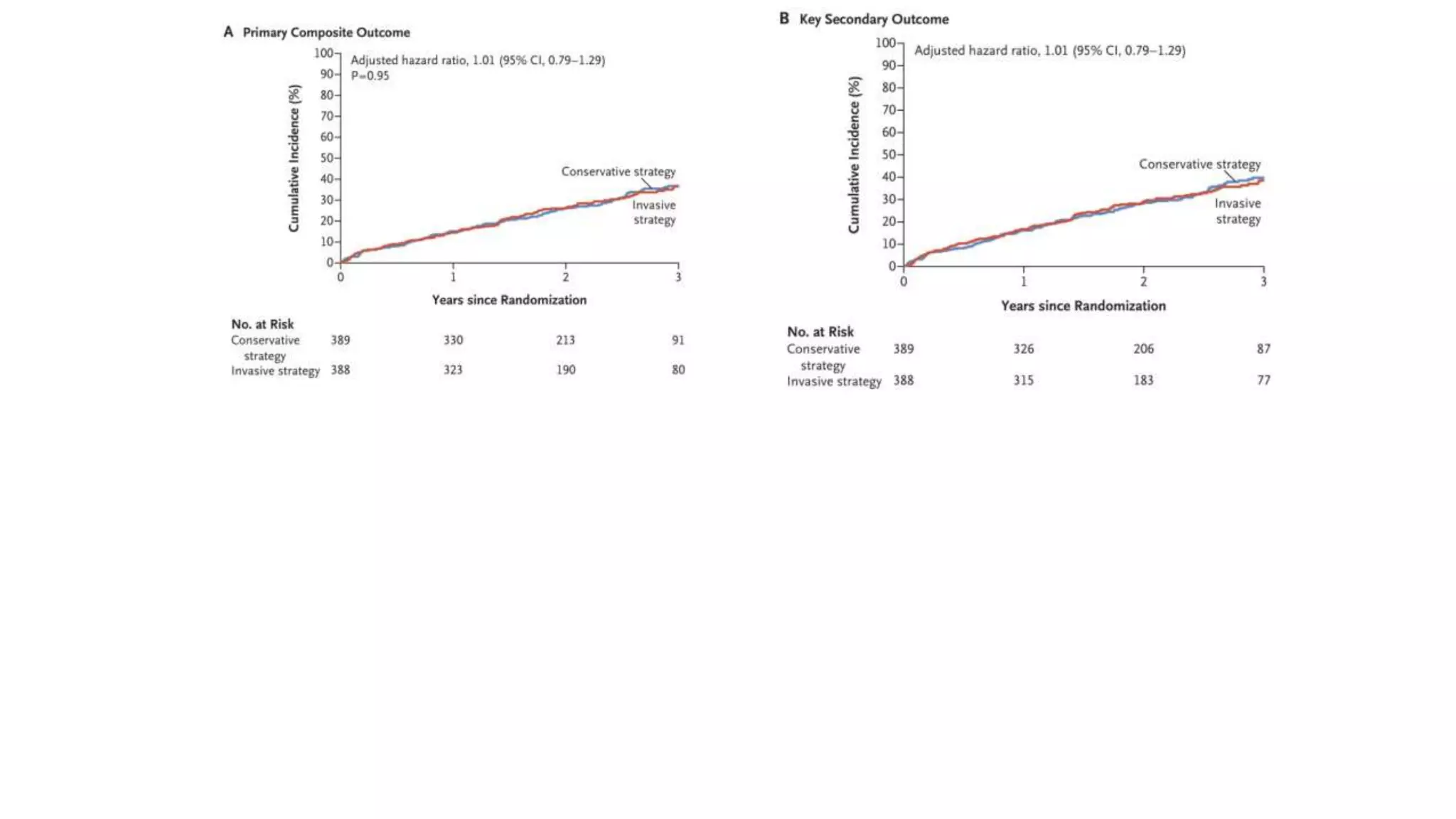
2) The trial found that an initial invasive strategy did not result in a lower rate of death or nonfatal myocardial infarction compared to an initial conservative strategy.
3) Limitations of the trial included exclusion of very symptomatic patients and lower than expected event rates, reducing the power to detect differences between strategies.






































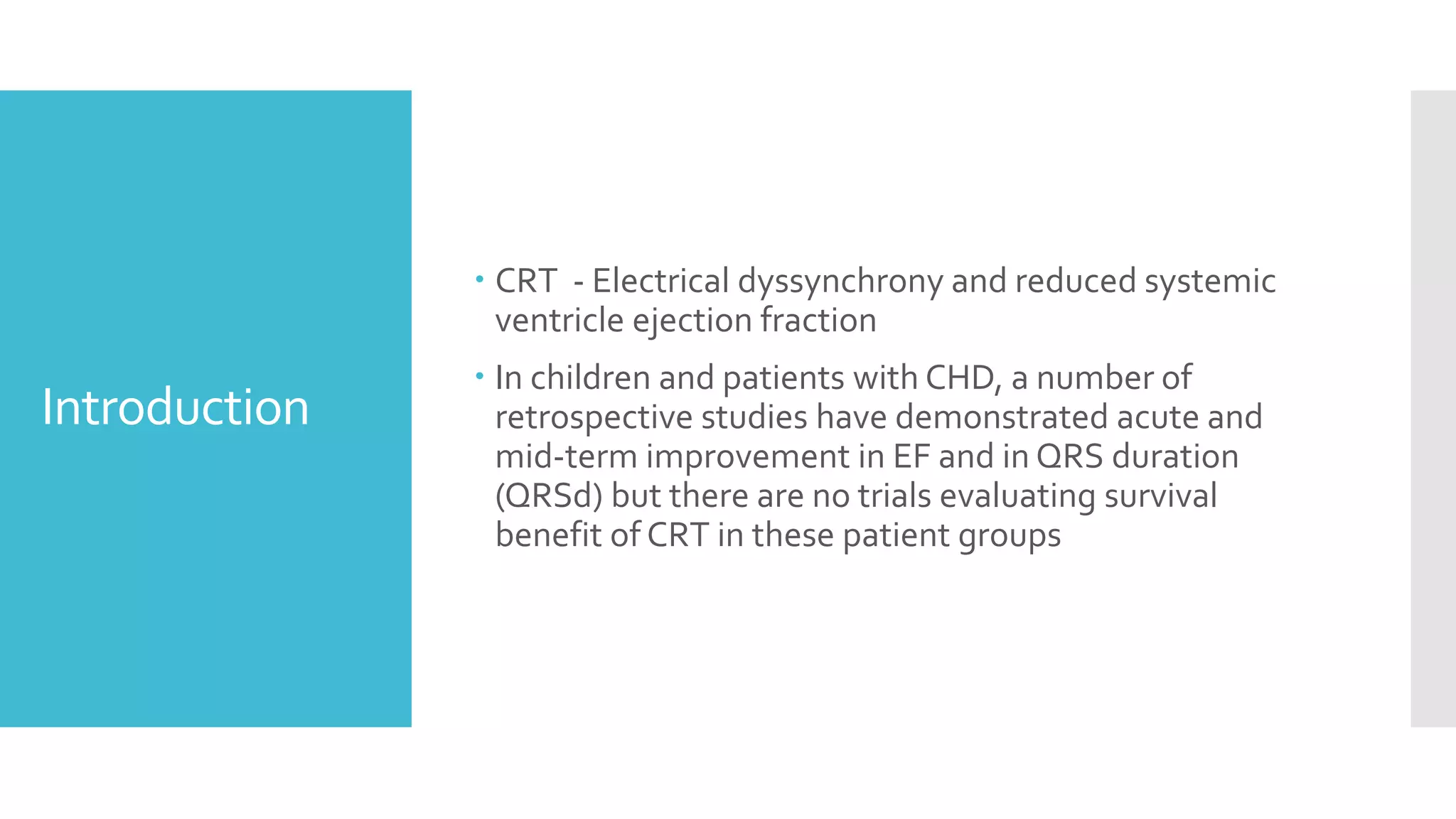

![Inclusion
Criteria
Age<21 years and/or CHD who underwent CRT at Lucile
Packard Children’s Hospital and Stanford Healthcare
between Jan 1st 2004 and December 31st 2017
Systemic SVEF <45%
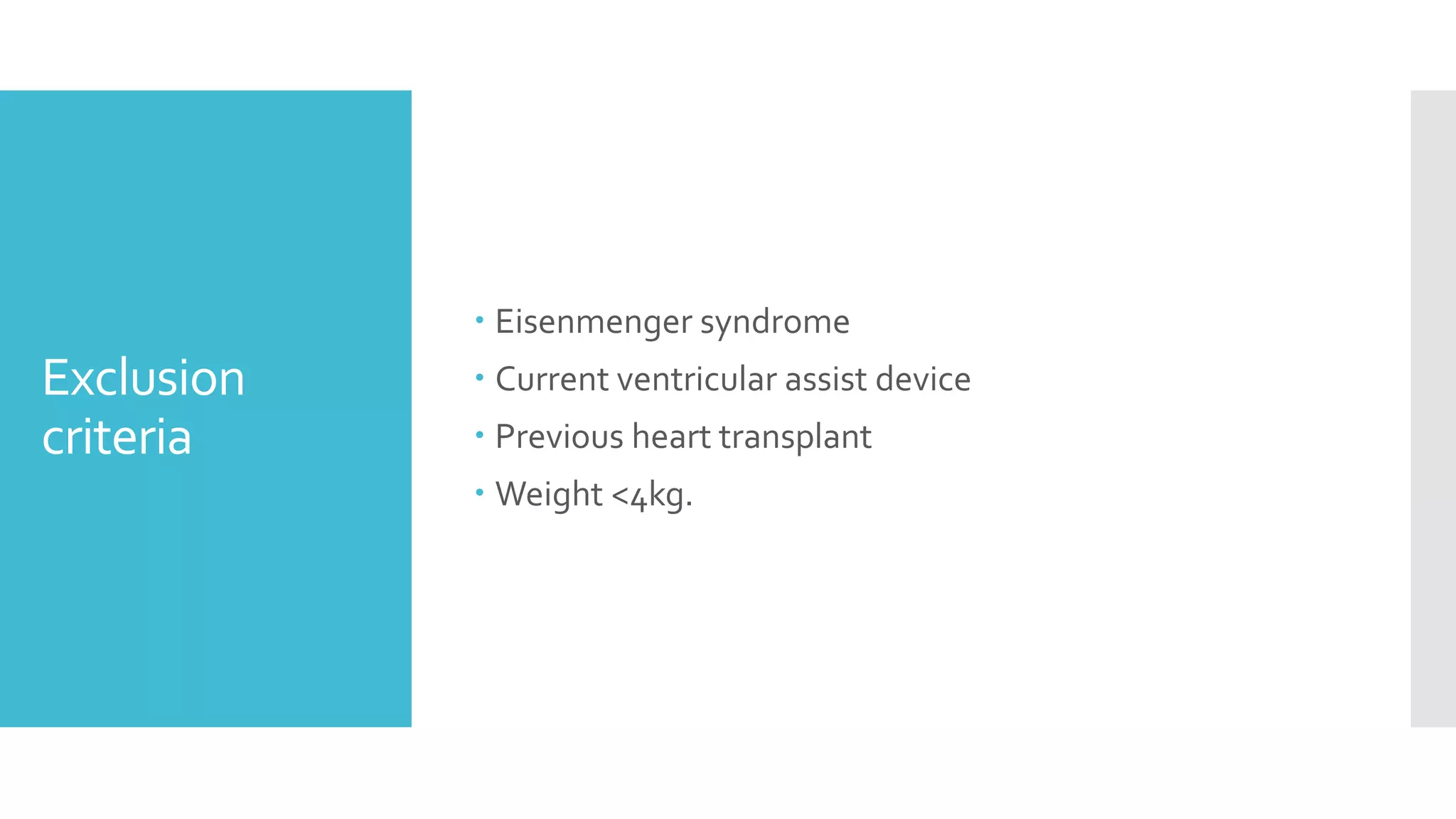
Symptomatic heart failure [defined as American Heart
Association Stage C or D]
Electrical dyssynchrony [defined as either a QRSd z-
score≥3 or, in paced patients, a ventricular pacing
burden (Vp) ≥40%]](https://image.slidesharecdn.com/journalreview27-04-20201-200430182742/75/Journal-review-27-04-2020-1-57-2048.jpg)