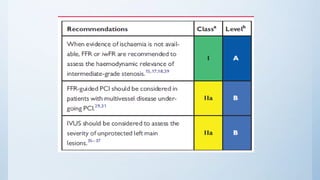
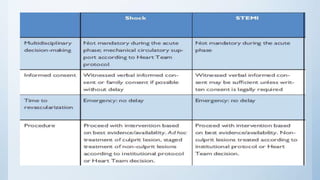
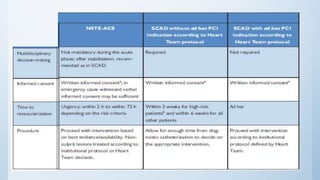
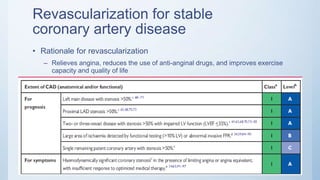
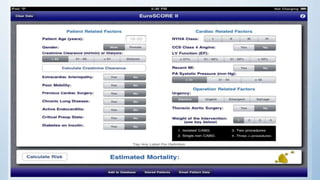
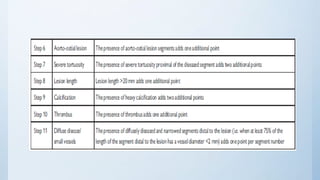
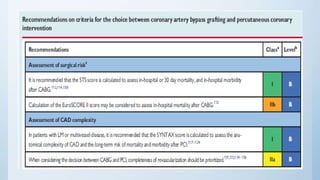
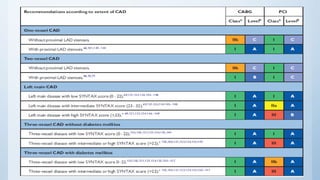
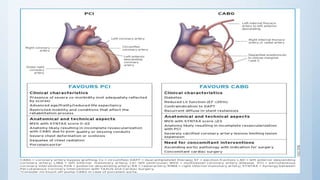
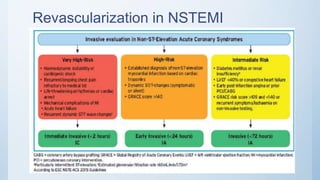
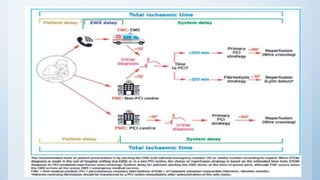
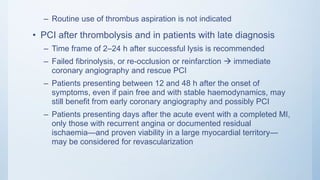
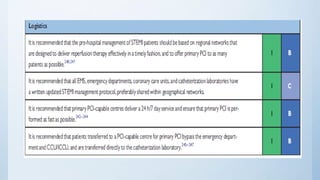
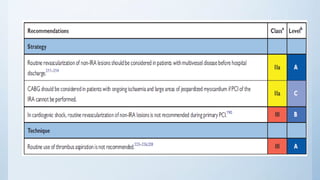
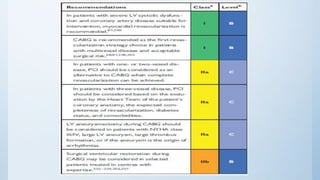
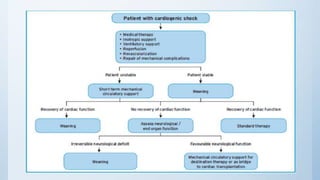
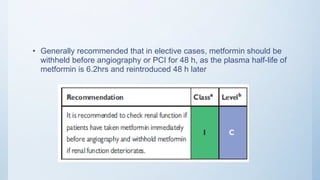
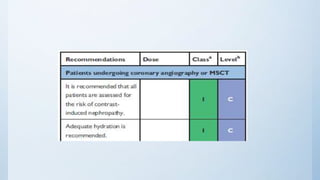
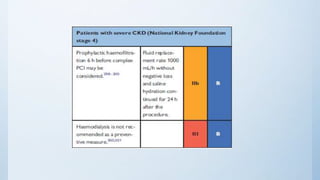
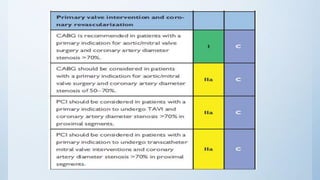
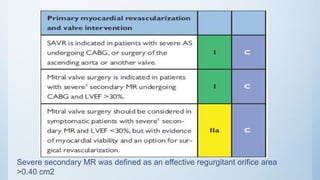
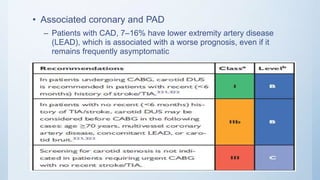
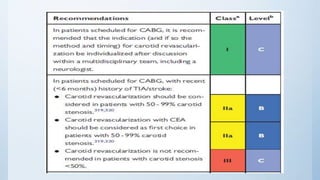
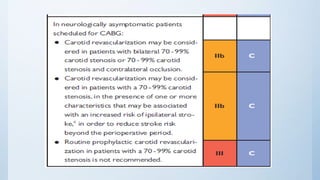
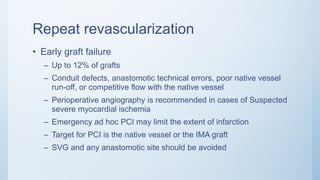
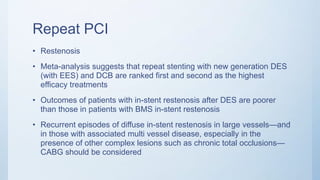
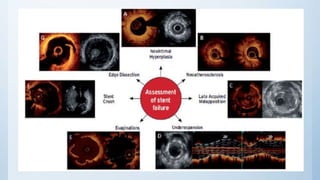
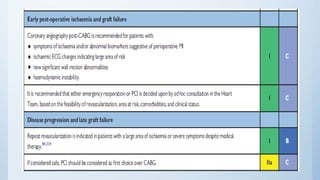
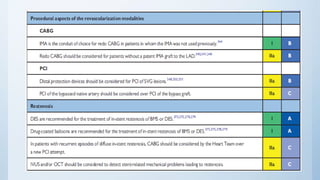
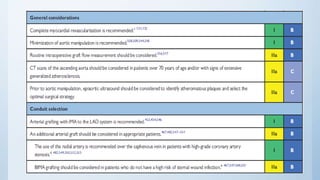
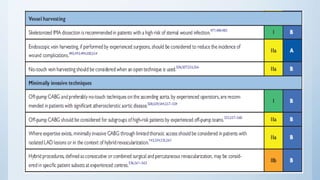
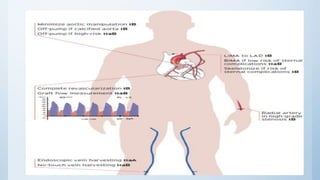
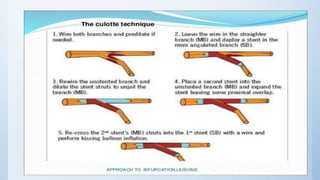
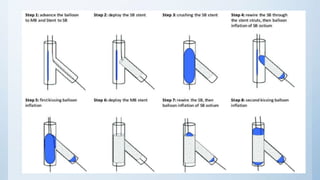
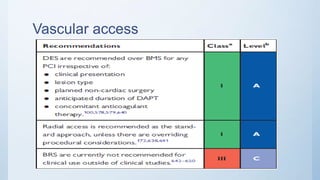
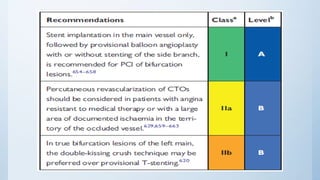
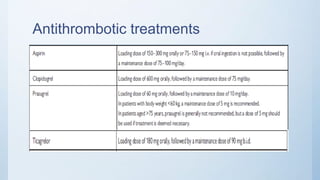
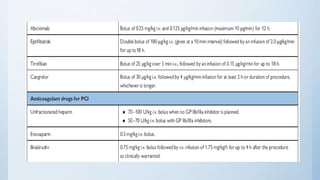
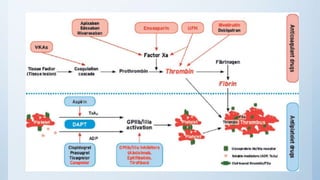
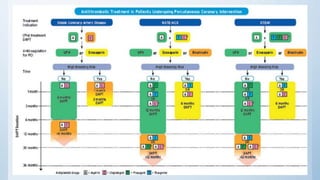
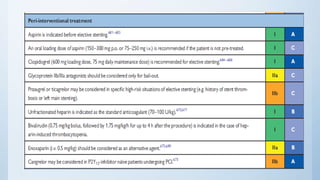
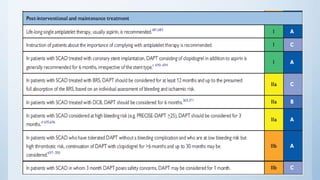
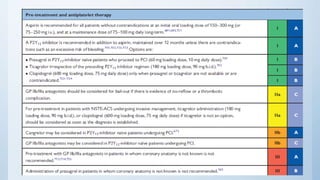
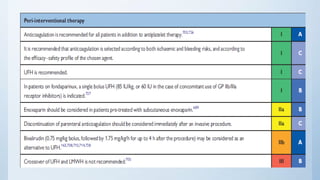
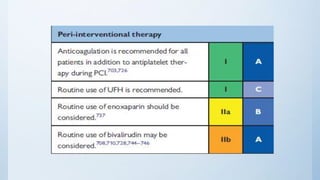
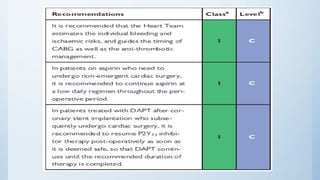
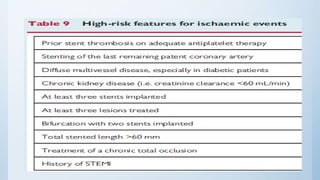
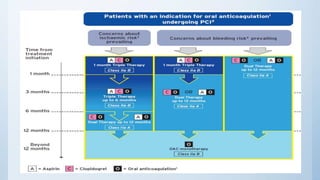
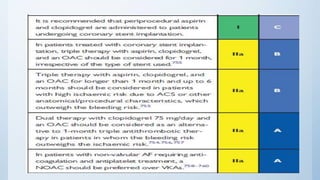
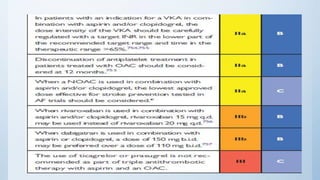
This document summarizes the 2018 ESC/EACTS Guidelines on myocardial revascularization. It discusses diagnostic tools to guide revascularization such as non-invasive imaging and invasive tools like fractional flow reserve. It also covers revascularization approaches for stable coronary artery disease, NSTEMI, STEMI, heart failure, diabetes, kidney disease and more. Procedural aspects of CABG and PCI are also summarized.