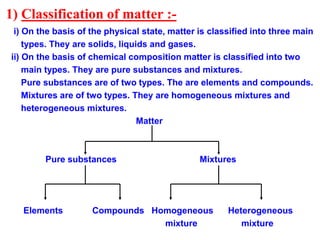
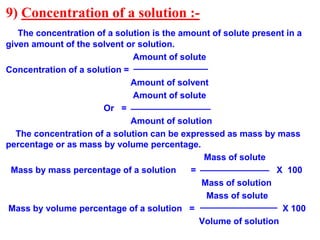
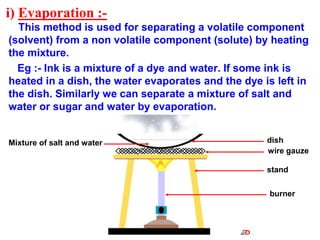
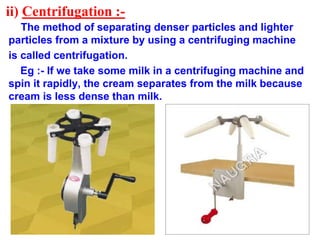
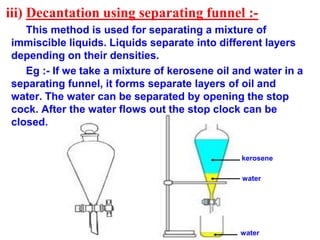
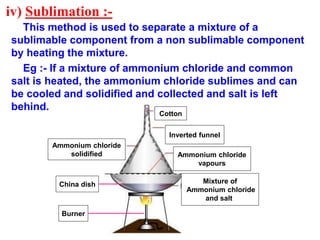
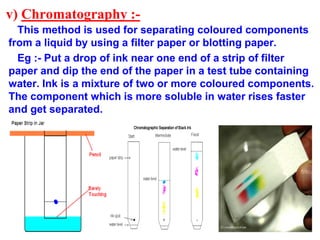
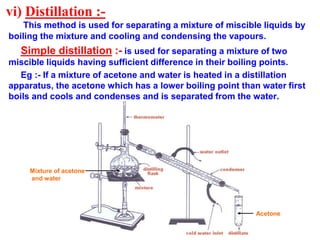
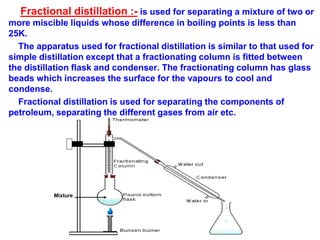
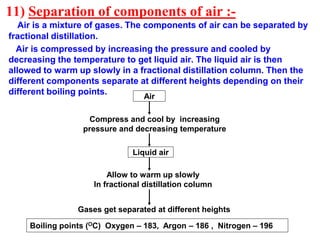
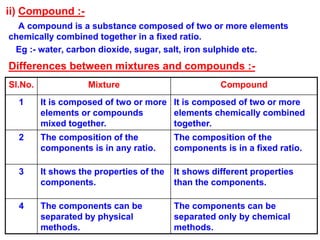
This document discusses the classification and composition of matter. It describes how matter can be classified based on physical state as solids, liquids or gases, and based on chemical composition as pure substances or mixtures. Pure substances are either elements or compounds, while mixtures can be homogeneous or heterogeneous. The key differences between pure substances and mixtures are outlined. Various techniques for separating components of mixtures are also explained, including evaporation, centrifugation, decantation, sublimation, chromatography, distillation and fractional distillation.