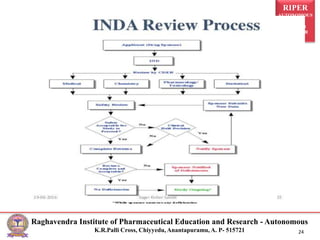
The document outlines the process and requirements for submitting an Investigational New Drug (IND) application to the FDA, which is necessary to initiate clinical studies for new drug products. It details the types and classifications of INDs, the components required in the submission, and the FDA review team involved in the assessment. Key sections of an IND include animal studies, manufacturing and clinical protocols, as well as pharmacology and toxicology information.