The document summarizes a study that tested the antibacterial properties and skin safety of nanosize silver colloid solutions applied to textile fabrics. The researchers found that the silver colloid killed over 99.99% of bacteria on the fabrics and maintained its antibacterial efficacy even after 10 washes. Skin tests on rabbits showed the silver colloid caused little to no irritation. The study demonstrated that nanosize silver colloids can be safely used as an effective antibacterial treatment for textiles.





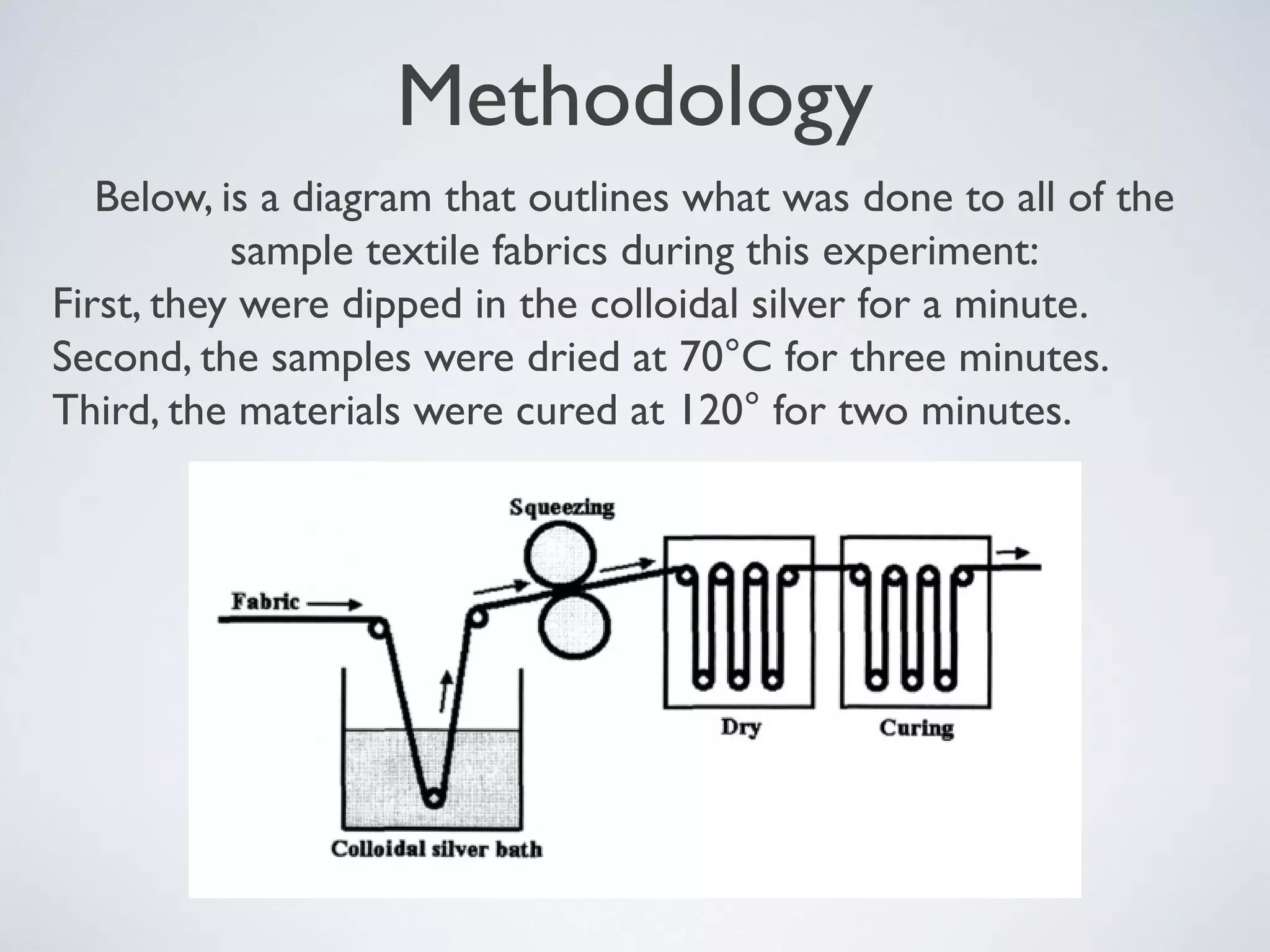
![Methodology
Materials
•nanosize (really small) silver colloidal solutions [2000ppm] - they
were dissolved in distilled water at room temperature [25°C]
•two kinds of fabrics were used - polyester and cotton
•to test the antibacterial effects of the colloidal silver, it was
evaluated against both Staphylococcus aureus and Escherichia coli
(two kinds of bacteria)
•The ATCC 6538 and 25922 were the microscope models that
were used to work with the bacteria and fabrics.Also, the ATCC
4352 was utilized when Klebsiela pneumoniae was assessed, rather
than the E. coli again.](https://image.slidesharecdn.com/presentation-101116120906-phpapp02/75/intro-to-research-6-2048.jpg)
![Methodology
Next, a skin irritation test was preformed on six white, male New
Zealand rabbits that were four months old. Each of them weighed
at least 2kg [4.4 pounds].This was done at room temperature.
One milliliter of the colloidal silver solution [100ppm] was applied
to the skin.The skin conditions of these rabbits were observed
after 24 hours and 72 hours.
Lastly, with the help of the various microscopes mentioned earlier,
the scientists viewed the colloidal silver particles, in order to
make sure that the substance completely covered the treated
fabrics (evenly distributed), which it did.This was done to make
sure that the data that was being collected was accurate.](https://image.slidesharecdn.com/presentation-101116120906-phpapp02/75/intro-to-research-8-2048.jpg)





