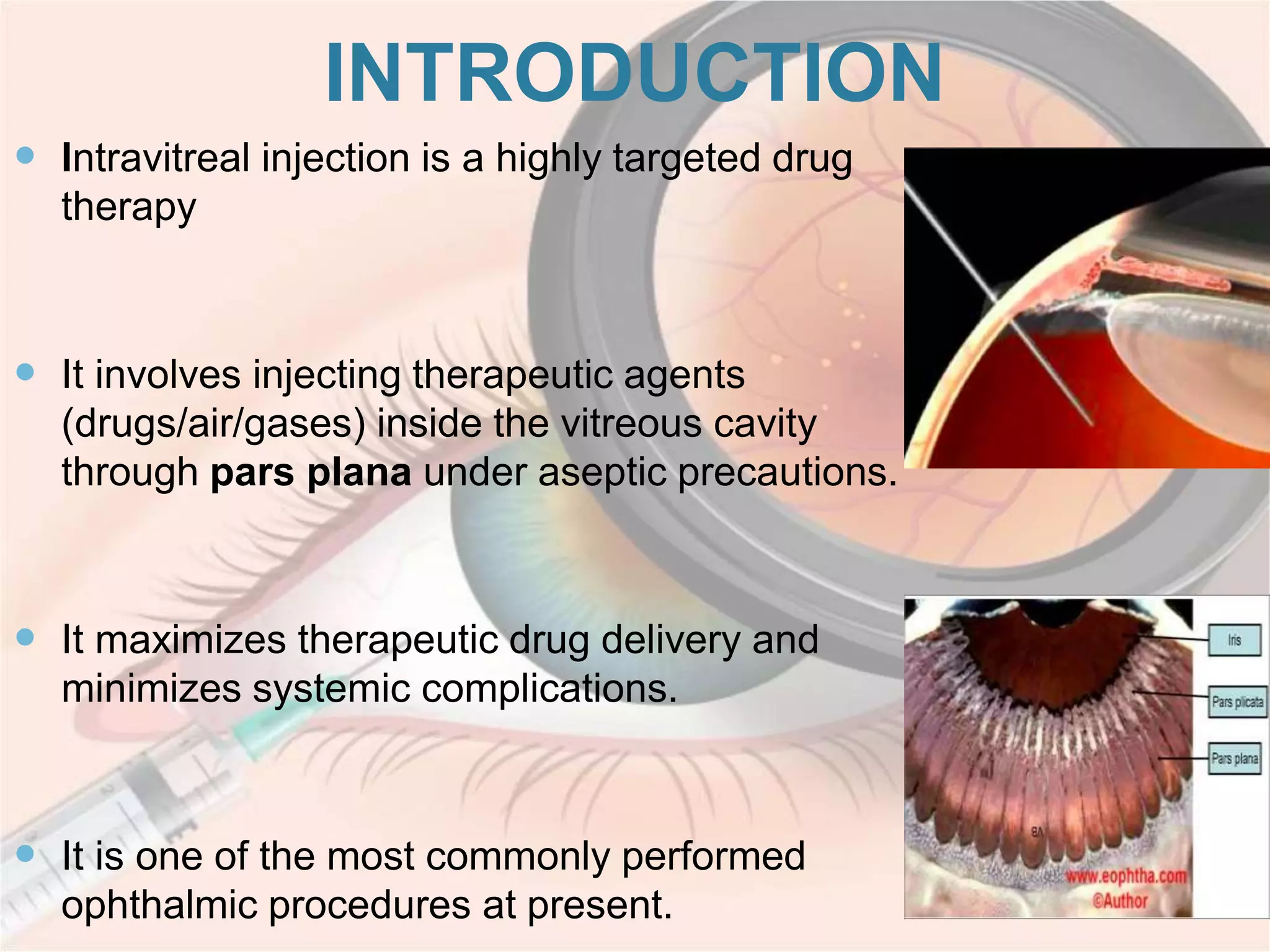
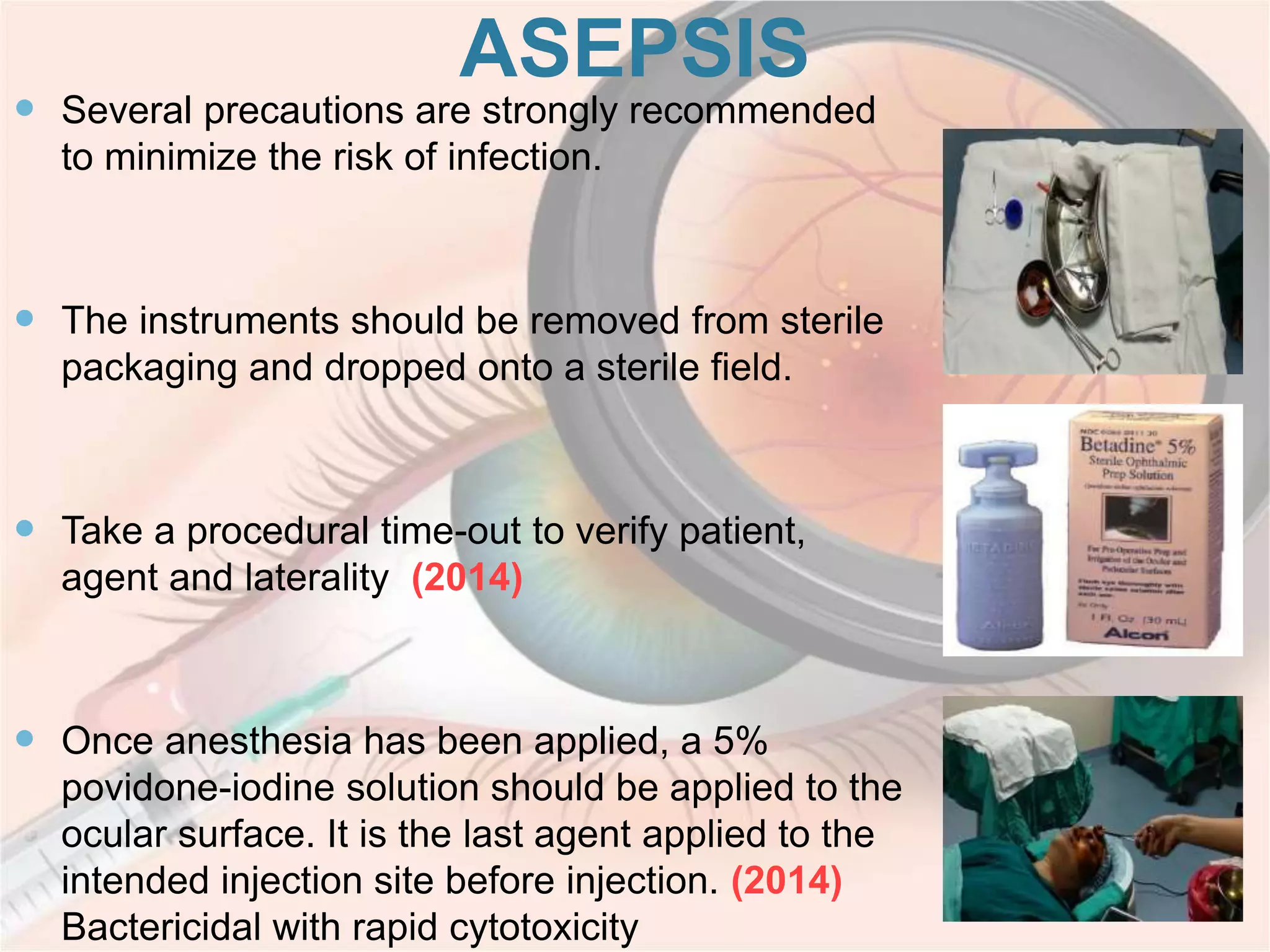
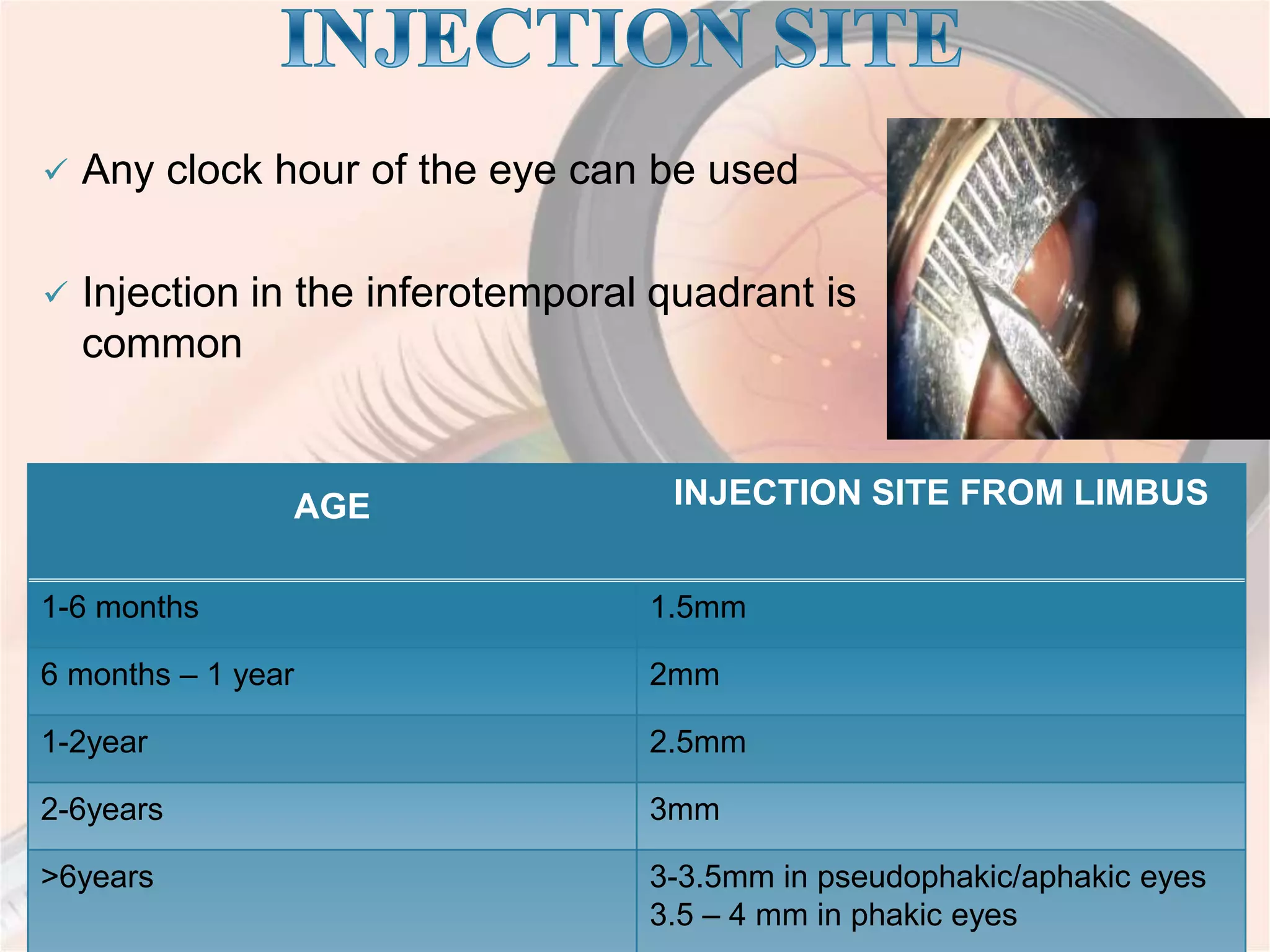
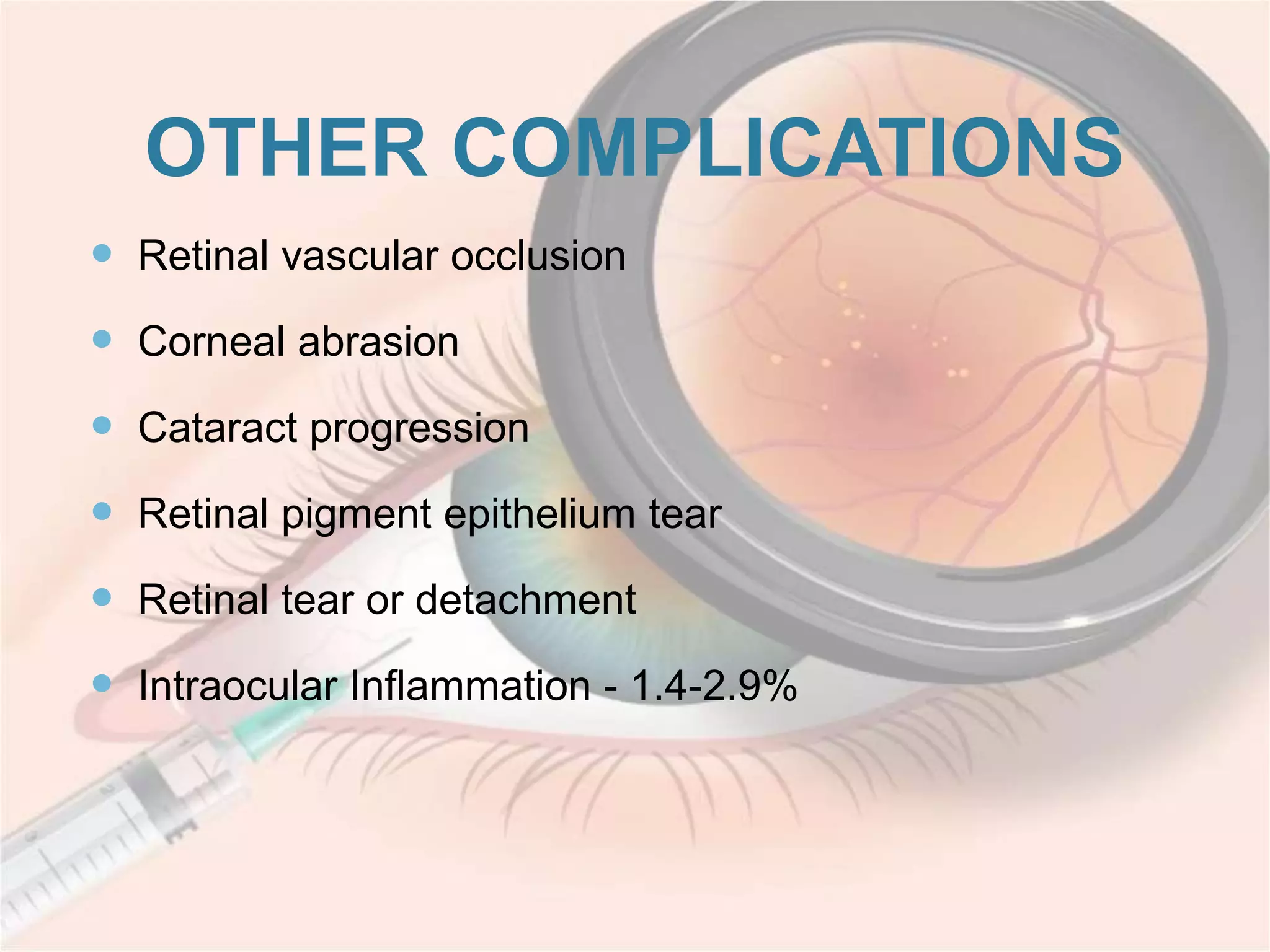
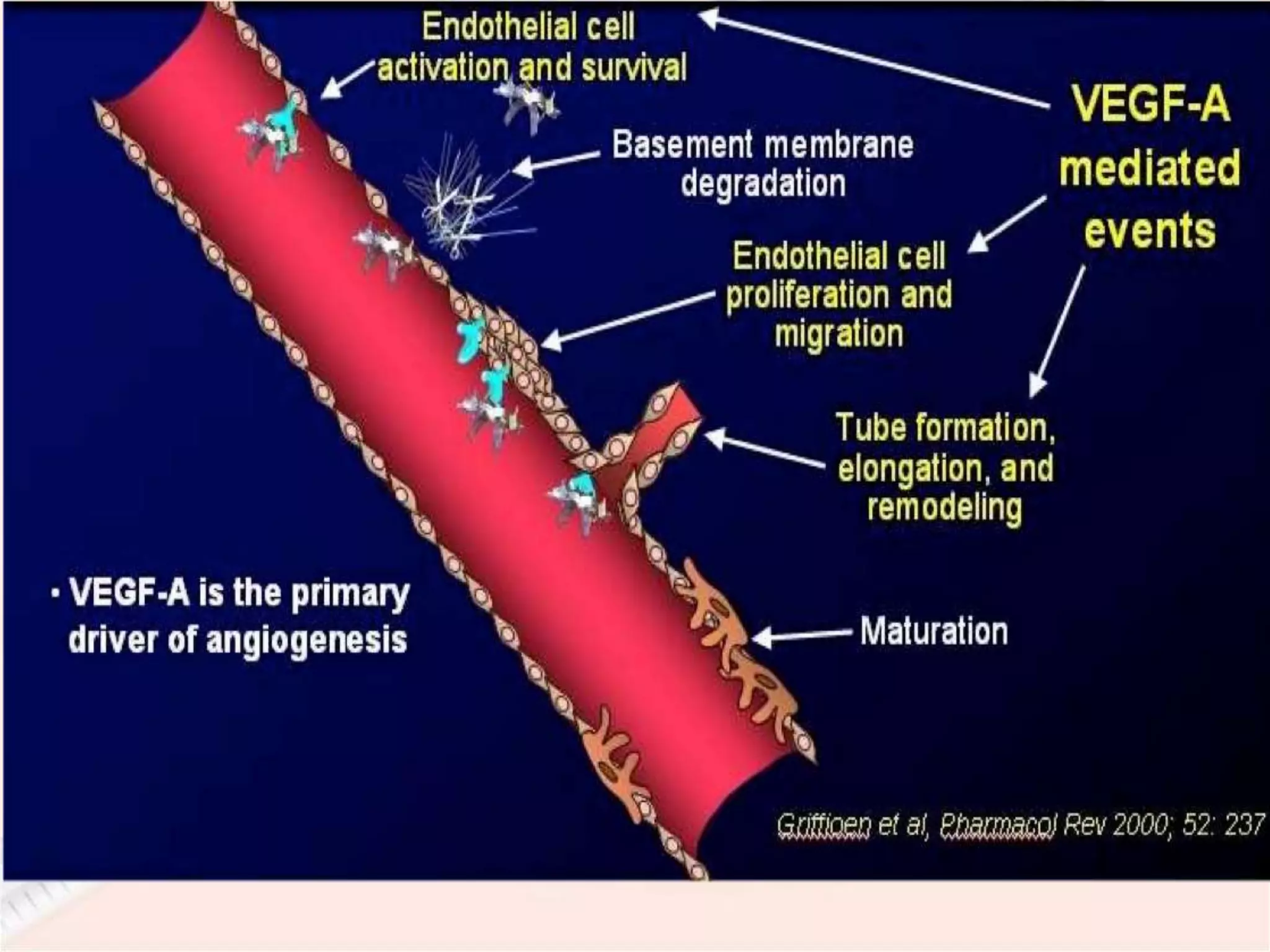
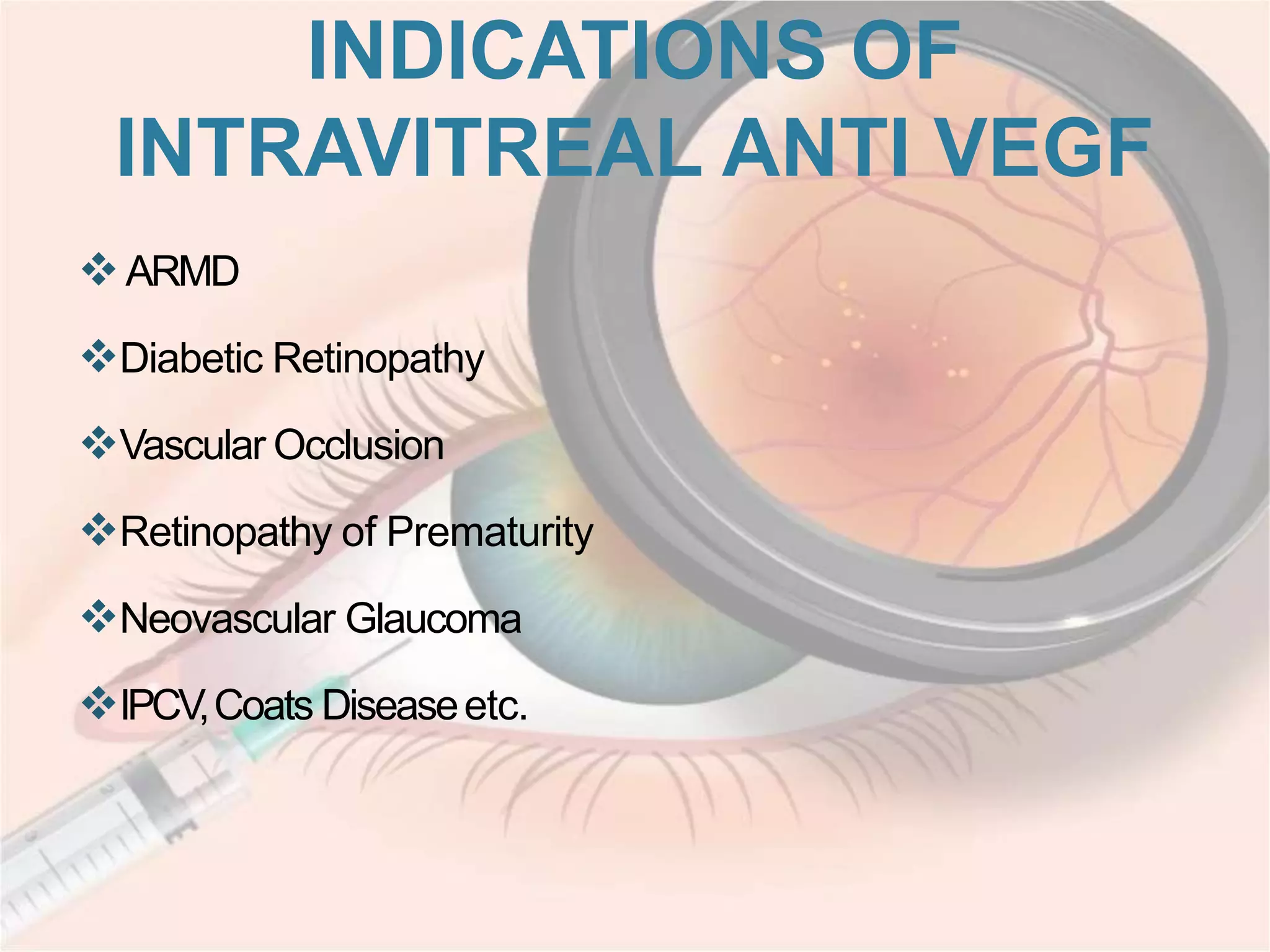
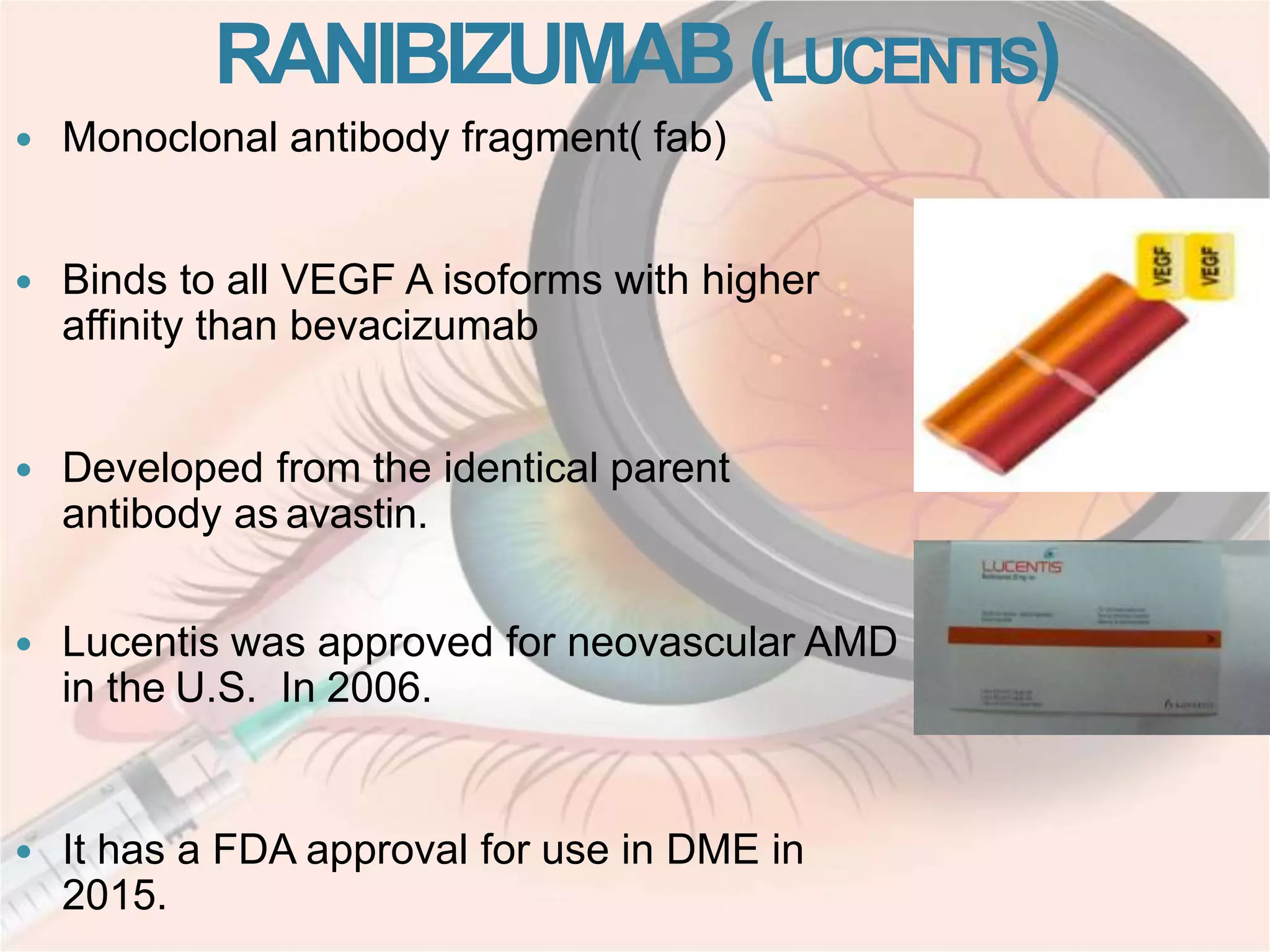
This document discusses intravitreal injections for drug delivery to the posterior segment of the eye. It begins by explaining the challenges of drug delivery due to the blood-ocular barrier and how sustained release systems and nano-particles were developed. It then provides details on the procedure of intravitreal injection, including indications, agents used, aseptic technique, complications, and anti-VEGF agents like bevacizumab, ranibizumab, pegaptanib, and aflibercept.