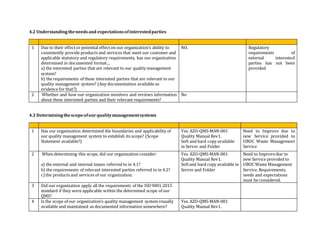
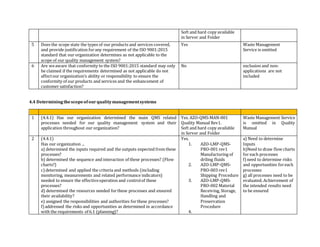
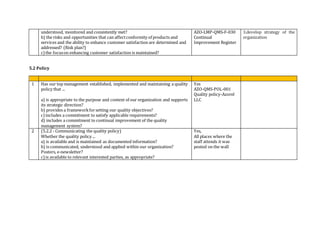
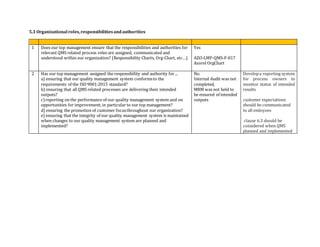
This document contains an audit checklist for an organization's quality management system. It assesses how the organization has addressed key requirements of ISO 9001:2015 related to understanding the context of the organization, needs and expectations of interested parties, scope of the quality management system, leadership and commitment to quality, and roles and responsibilities. The audit found the organization needs to improve in areas like documenting processes and risks, updating procedures for new services, developing performance indicators, and ensuring top management involvement through activities like internal audits and management reviews.