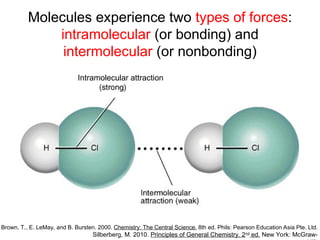
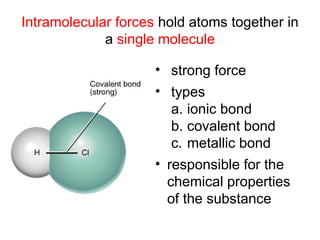
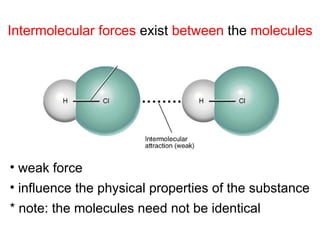
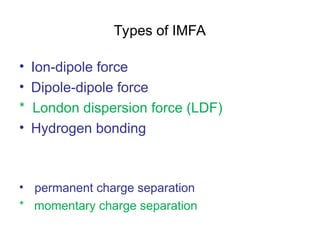
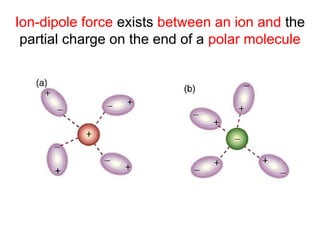
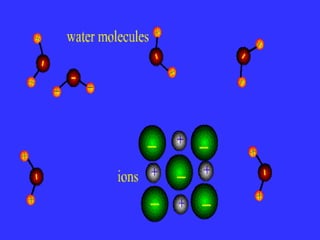
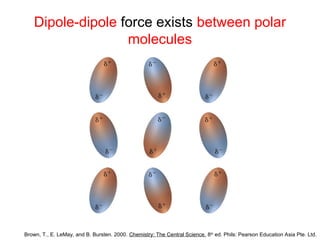
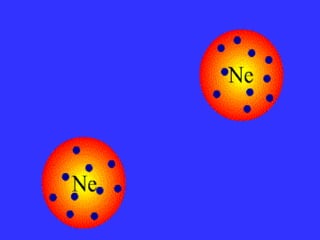
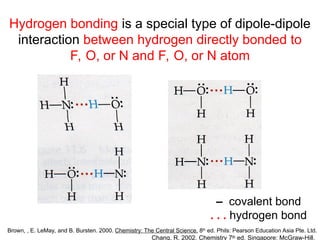
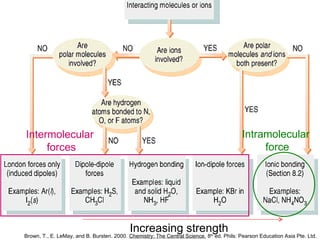
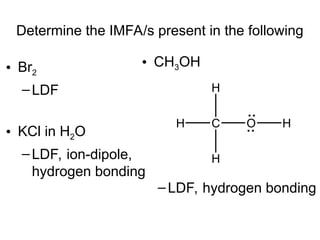
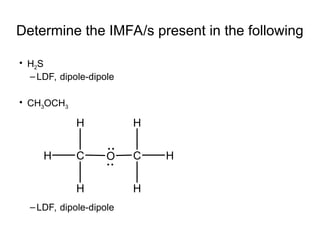
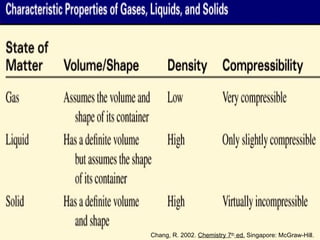
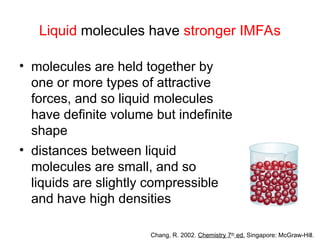
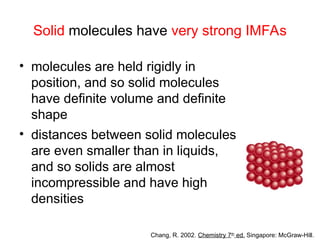
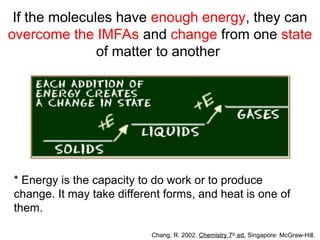
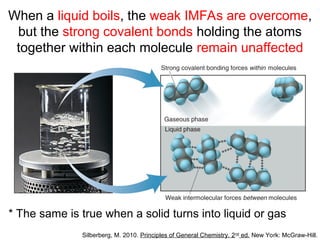
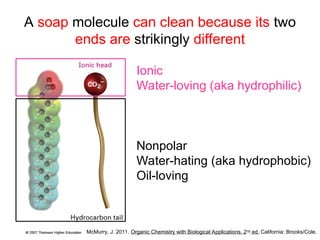
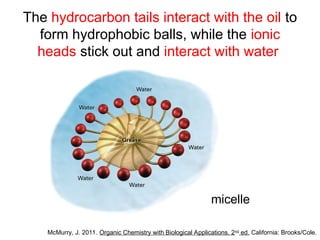
The document discusses the different types of intermolecular and intramolecular forces that affect molecular interactions, highlighting strong intramolecular forces (ionic, covalent, and metallic bonds) and weaker intermolecular forces (ion-dipole, dipole-dipole, London dispersion, and hydrogen bonding). It describes how these forces influence the physical properties and states of matter, with solid, liquid, and gas exhibiting varying strengths of intermolecular attractions. Additionally, the document illustrates practical examples of these forces, including the behavior of soap molecules in water and the insulating properties of ice on lakes.