Embed presentation
Download to read offline

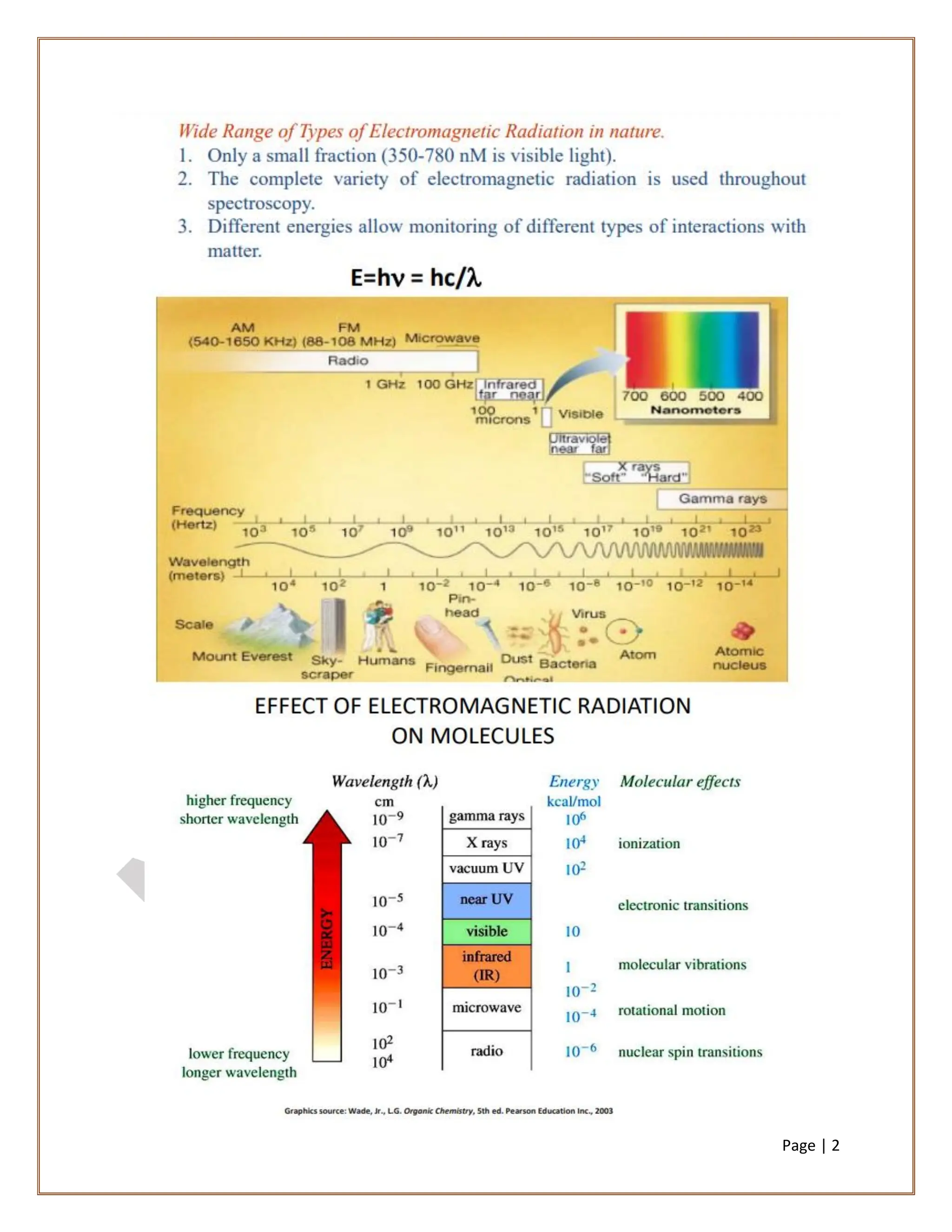
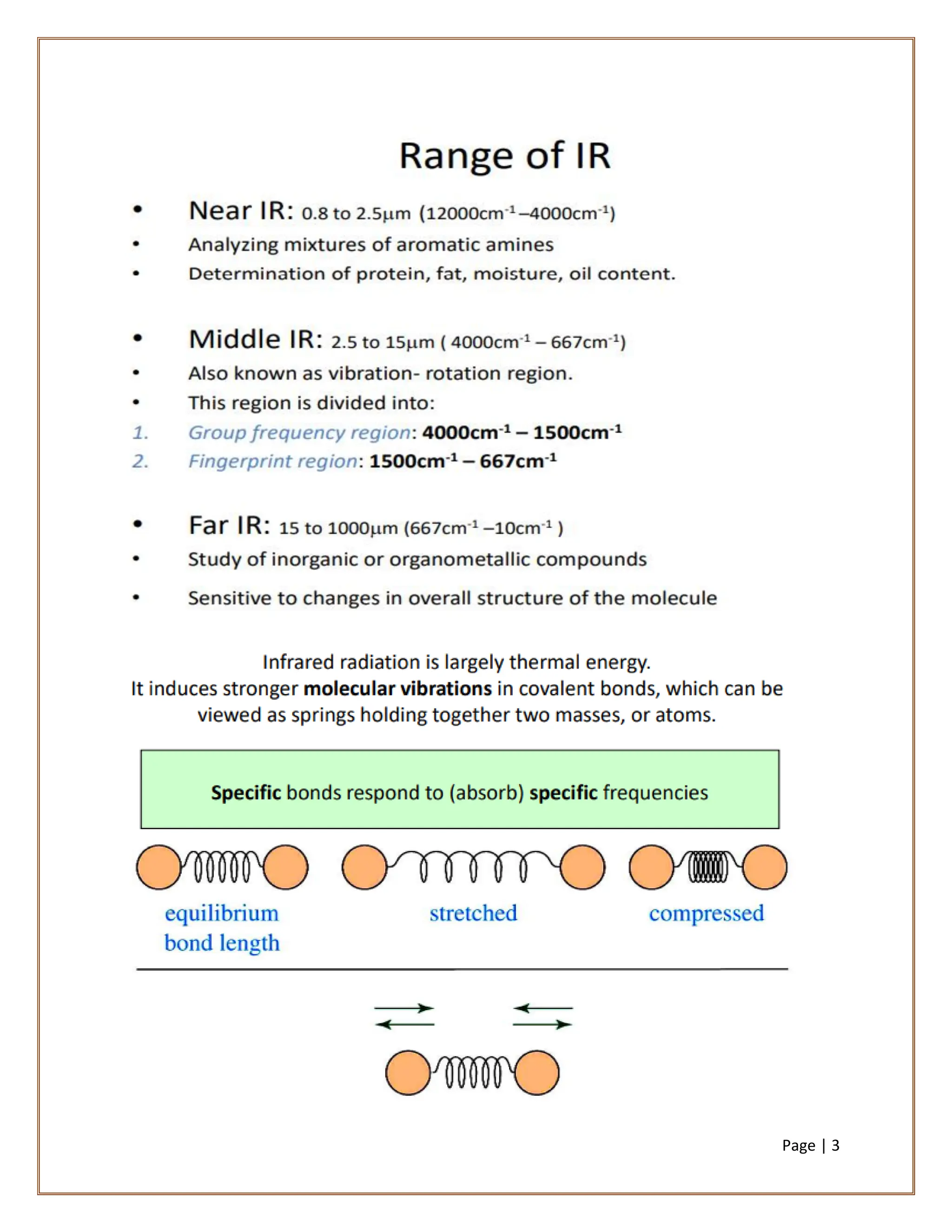
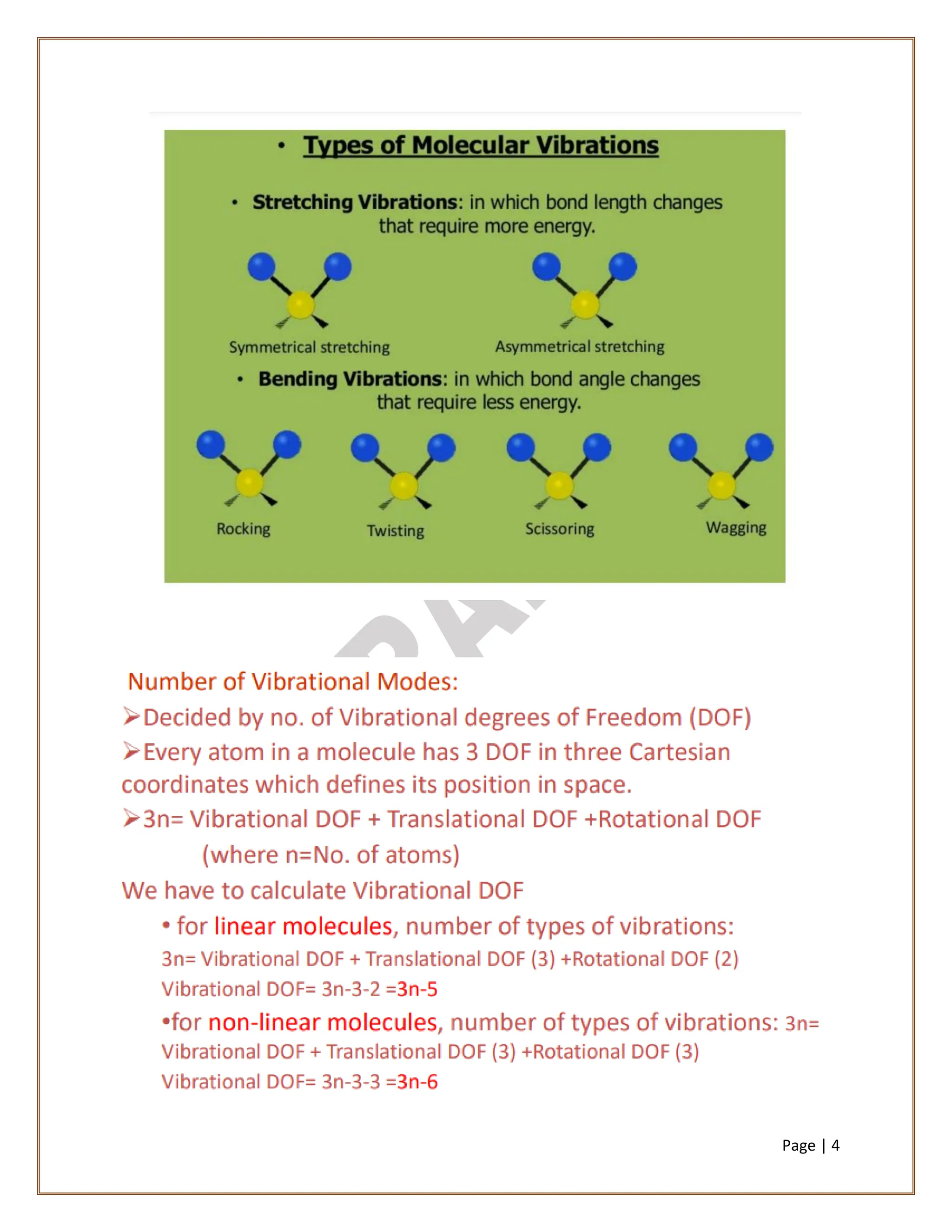
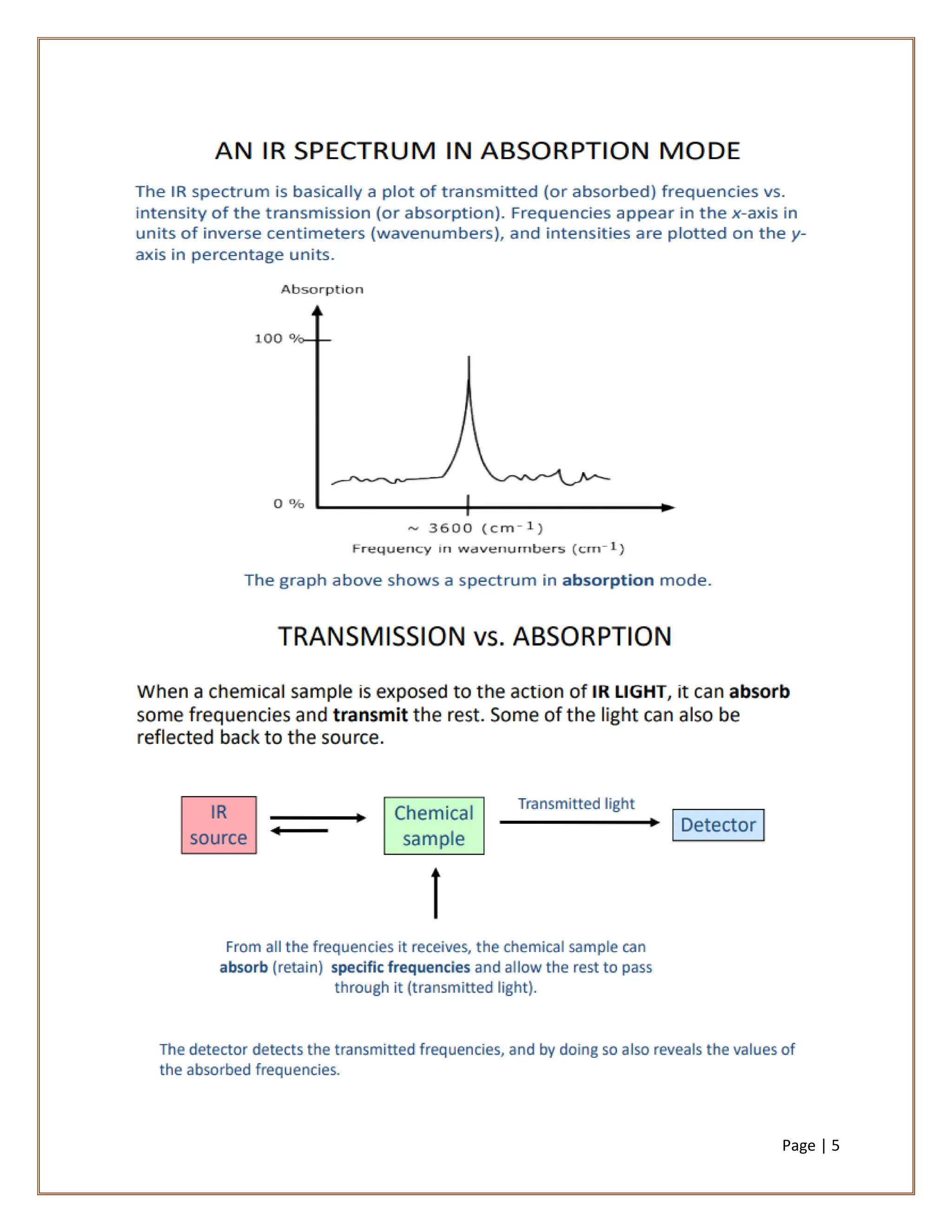
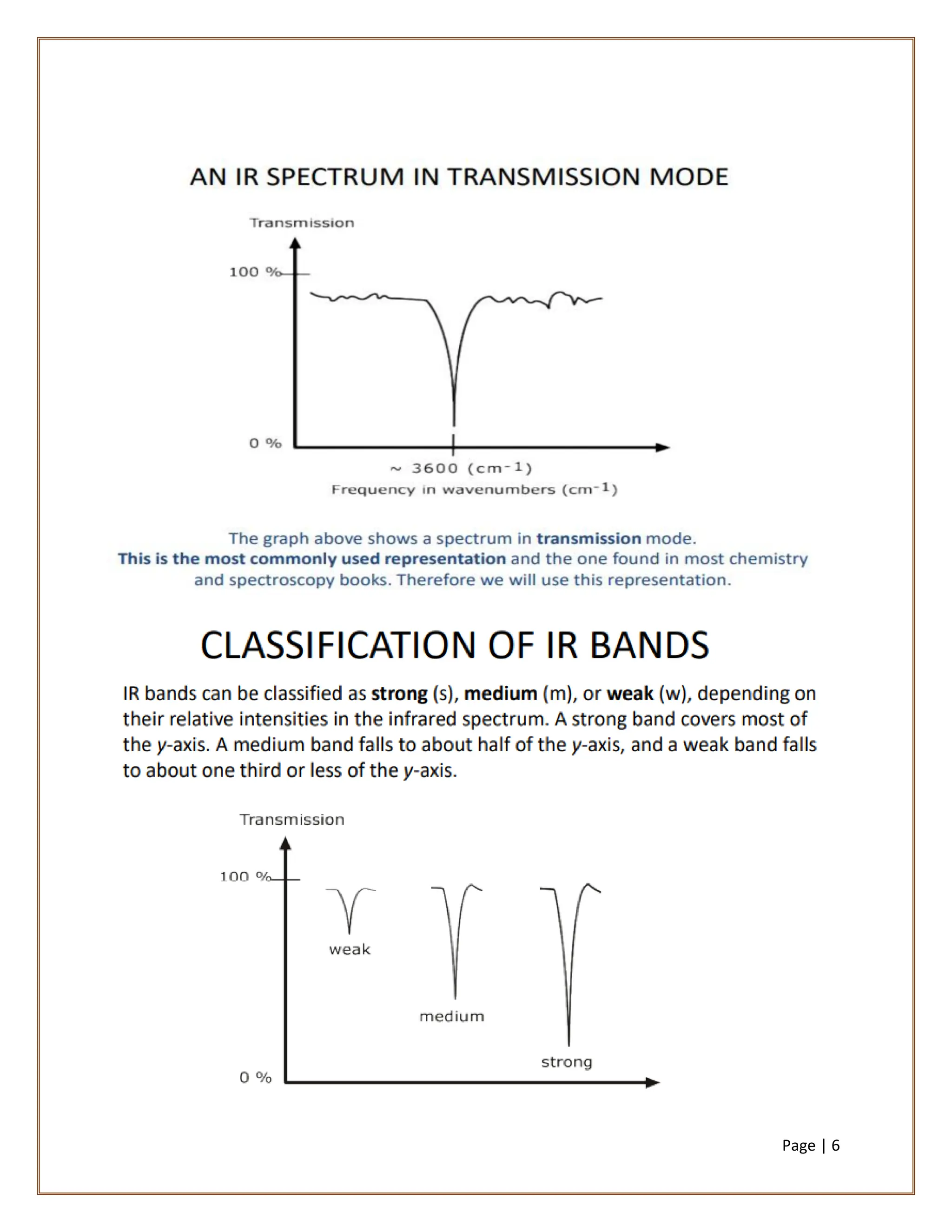
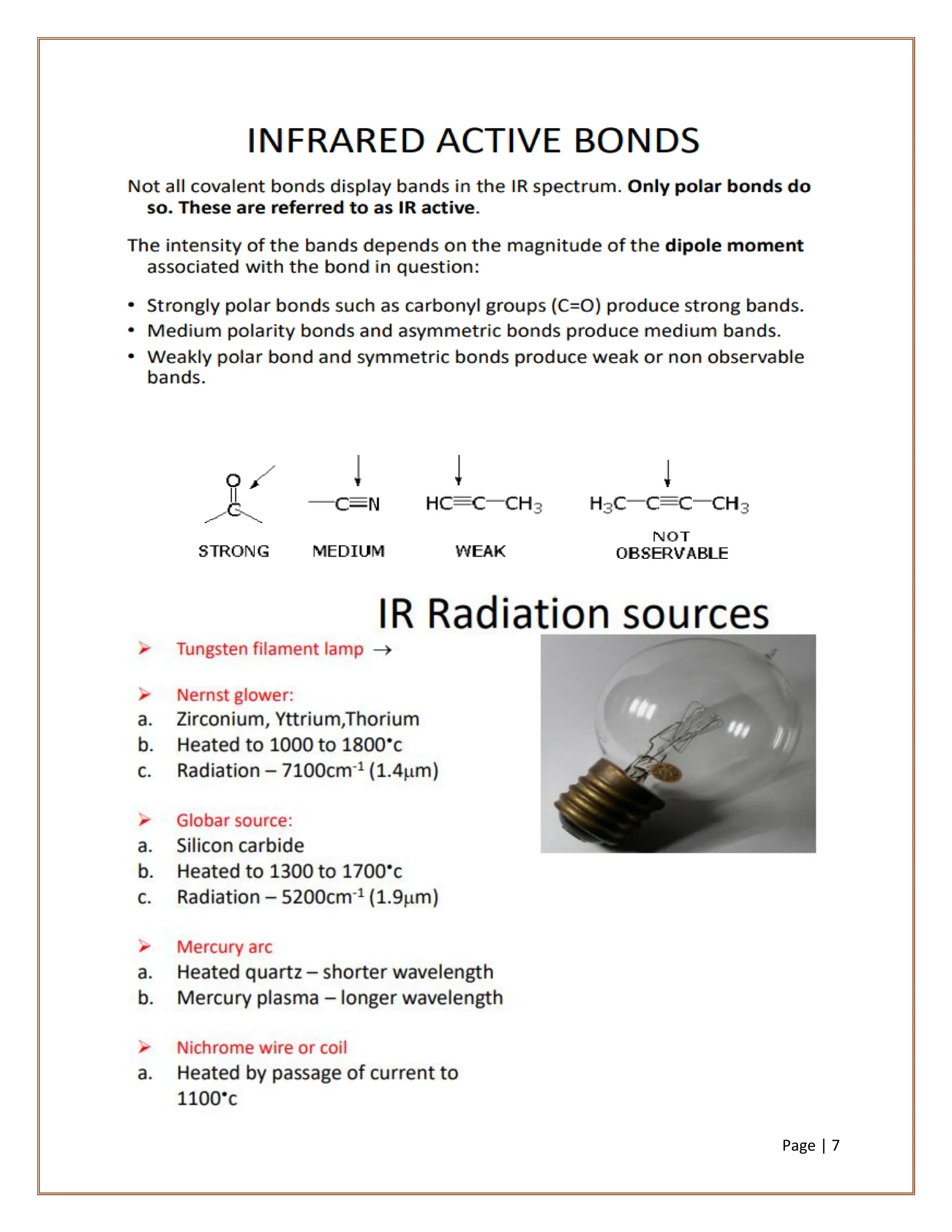
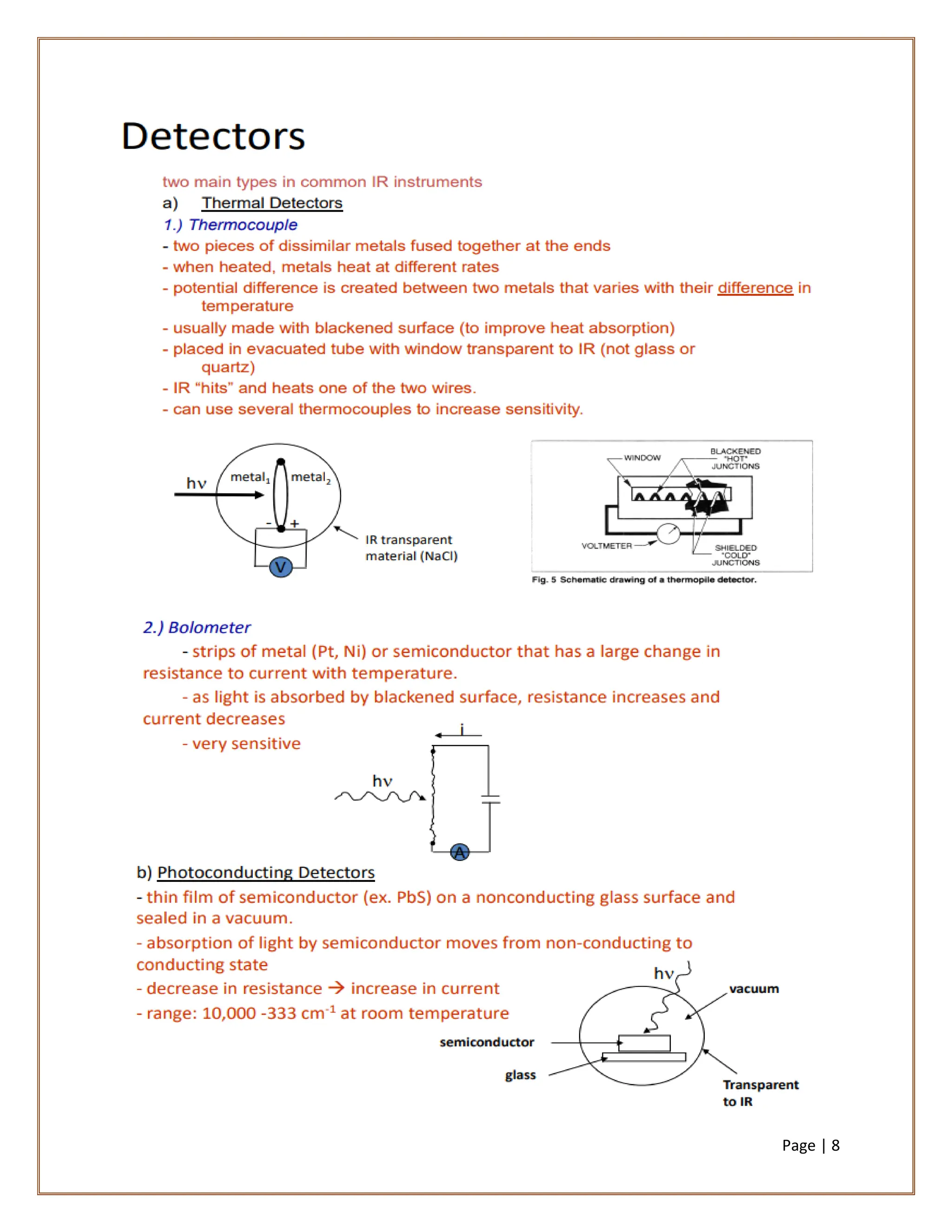


Infrared spectroscopy is a critical technique for analyzing the structure of organic compounds, offering a spectrum rich in absorption bands compared to UV spectroscopy. It involves the excitation of molecules to higher vibrational levels when they absorb infrared radiation, with only those transitions that change the dipole moment being observed. This technique highlights that vibrational transitions linked to certain bonds (e.g., C=O, N-H) are infrared active, while others (e.g., C-C bonds in symmetrical alkenes) are not.








