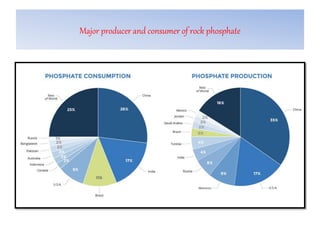
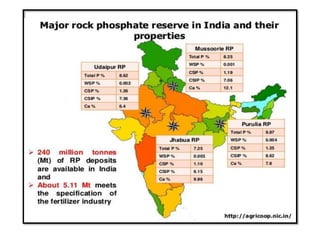
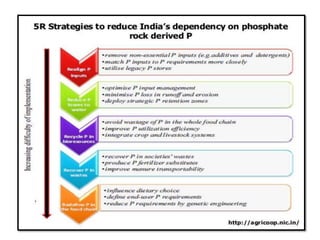
The document discusses the use and efficiency of rock phosphate (RP) in agriculture, highlighting its origins, reactivity, and application methods. It outlines the benefits of RP, including cost-effectiveness and gradual phosphorus release, while also identifying factors affecting its effectiveness, such as soil properties and management practices. Additionally, it presents various strategies to improve RP efficiency, including biological, chemical, and physical methods.